Targeting ligand molecule, preparation method thereof and drug delivery system
A ligand molecule and liver-targeting technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients. Balance, reduced efficiency of liver targeting, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
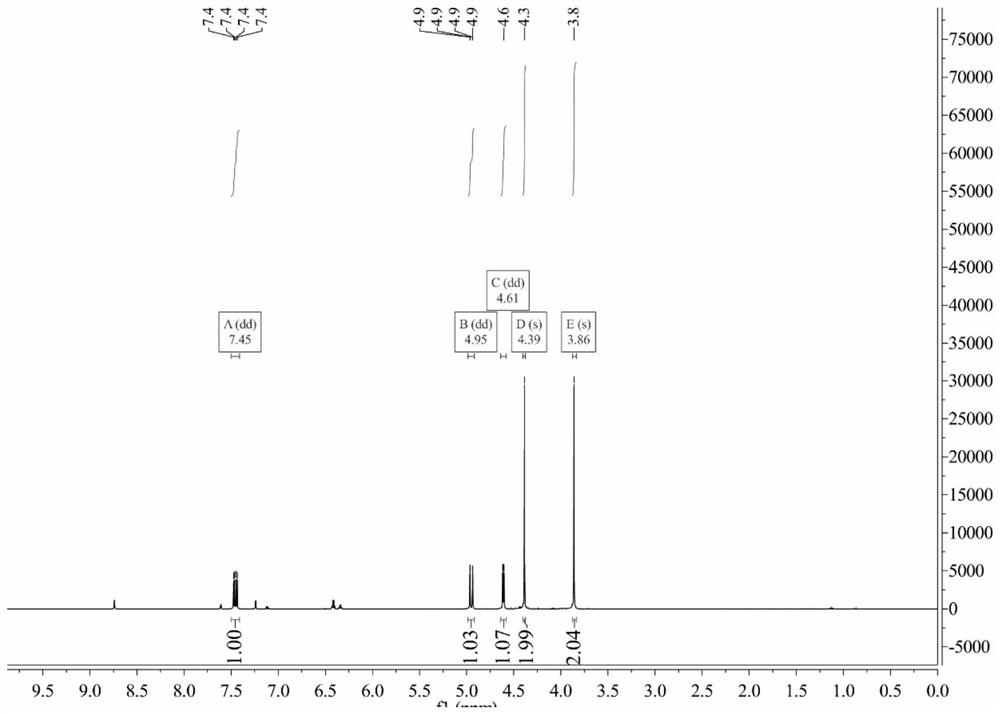
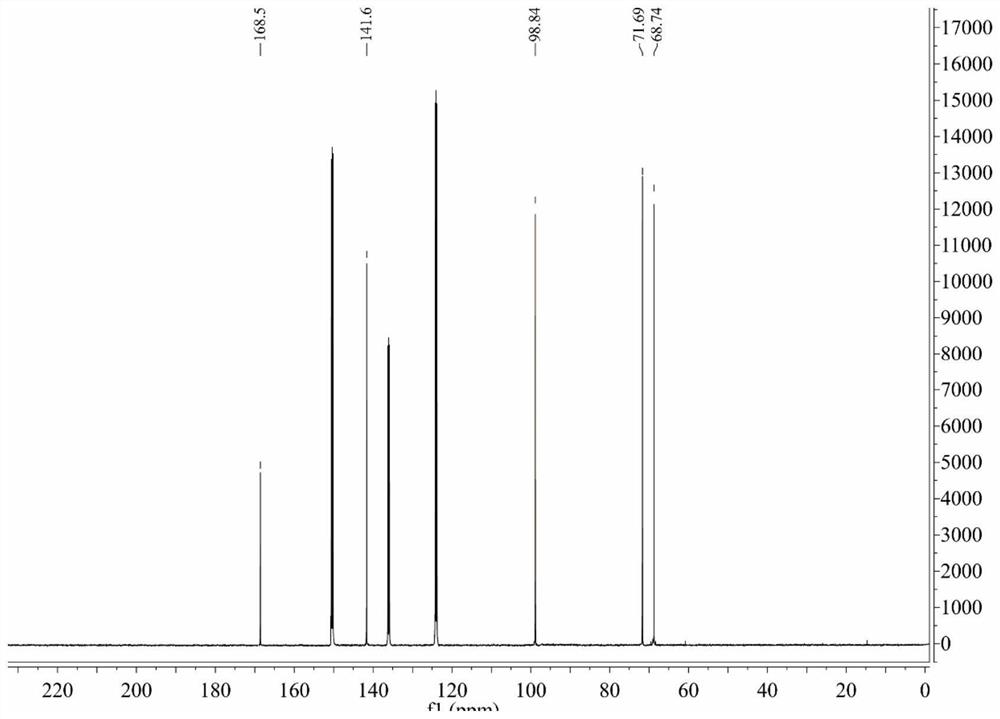
[0078] The chemical synthesis route of embodiment 1CHS-DIO-SA-GalNAc
[0079] The synthesis steps are as follows:
[0080]
[0081] Synthesis of step 1 intermediate 1,3,6-dioxa-(divinyl suberate) (3,6-dioxa-(divinyloctanedioate))
[0082] Weigh 28mol of 3,6-dioxaoctandioic acid, 5.6mol of vinyl acetate, 0.028mol of mercury acetate and a small amount of copper acetate into a 1000mL three-necked flask. Heat and stir the three-necked flask containing the above substances in a constant temperature water bath at 50°C, add 0.5 mL of concentrated sulfuric acid dropwise after 10 min, and react for 8 h. After the reaction was completed, about 4g of sodium acetate was added to fully shake to neutralize the sulfuric acid. After the excess vinyl acetate was distilled off by water pump under reduced pressure, the remaining blue liquid was chromatographed on a silica gel column and eluted isocratically with petroleum ether-ethyl acetate (9: 1), and the pure product was isolated with a ...
Embodiment 3
[0099] The preparation of embodiment 3 drug-carrying system liposome
[0100] 3.1 Preparation of doxorubicin liposomes
[0101] Preparation of Doxorubicin Liposomes by Gradient Loading Method [18] . Mix HSPC and CHS according to a certain ratio (see Table 1), dissolve in chloroform, place in a water bath rotary evaporator at 55°C, evaporate the chloroform to dryness until a layer of lipid film is formed on the inner wall of the bottle, and dry overnight in a vacuum desiccator (12 h), then add ammonium sulfate solution (300 mmol / L) for hydration, stir slowly at 55 ° C, incubate for 1 h, and then use a high-pressure extruder to pass through 0.1 and 0.05 μm polycarbonate membranes 10 times each to obtain the blank lipid body. Then pass the blank liposomes through a Sephadex G-50 column to remove unencapsulated ammonium sulfate, and finally add DOX solution, and incubate at 65°C for 1 hour to obtain DOX-loaded ordinary liposomes (CL-LP DOX).
[0102] Table 1 Common lipid doxor...
Embodiment 4
[0110] The characterization of embodiment 4 liposome
[0111] 4.1 Liposome particle size and Zeta potential measurement
[0112] Take 100 μL of the above liposome solution, dilute to 2 mL with normal saline, mix well, and analyze its particle size distribution, Zeta potential, and polymer dispersion index (PDI) using a laser scattering particle size analyzer. The experimental data are expressed as Statistical software SPSS 22.0 was used for data analysis, and the mean comparison of particle size, PDI and Zeta potential among the groups was performed by analysis of variance. The results are shown in Table 4.
[0113] Table 4 liposome characterization ( n=3)
[0114]
[0115]
[0116] (-) represents anionic liposomes containing DSPG-Na. Leakage rate < 3%
[0117] ** indicates that the particle size of GalNAc-DIO-LP DOX group is significantly different from that of GalNAc-LP DOX group (P<0.01); *** indicates CL-LP DOX, GalNAc-LP DOX, Gal-LP DOX, Lac-LP DOX , GalNAc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com