Method for synthesizing deuterated compound in aqueous phase solvent
A technology of deuterated compounds and water-phase solvents, applied in the field of isotope labeling chemical synthesis, can solve the problems of difficult acquisition of deuterium sources, decreased deuteration rate, difficult operation, etc., and achieves the effects of good applicability, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Generally speaking, the synthetic method of deuterated compound of the present invention comprises the following steps:
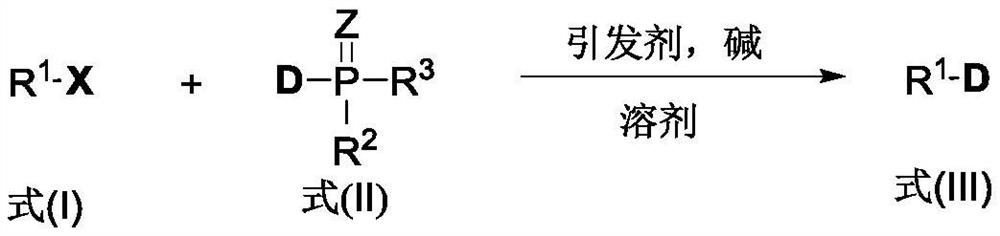
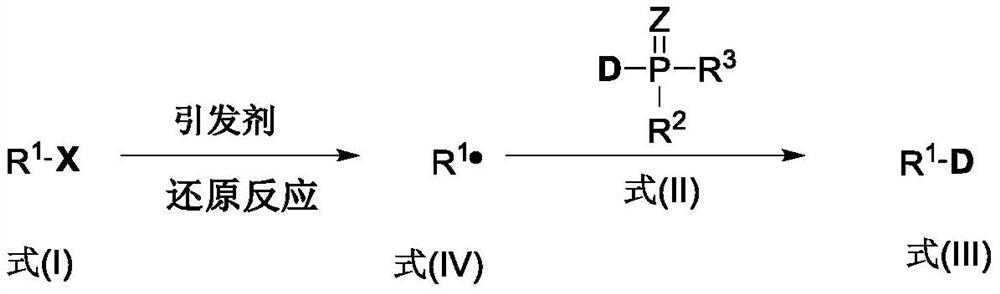
[0042] (1) mix the compound shown in formula (I), the deuterium source containing phosphorus-deuterium bond shown in formula (II), initiator, alkali and water phase solvent, obtain the reaction raw material mixed system to react; Reaction general formula is:
[0043]
[0044] Among them, R 1 All are selected from at least one of alkyl, aryl, and acyl; X is selected from at least one of iodine, bromine, chlorine, diazo, and xanthate (shown in formula I as halogenated, heavy Nitrogen salt or xanthate (Xanthate)); Z is selected from at least one of oxygen atom and sulfur atom; R 2 , R 3 At least one of salt compounds selected from deuterium atoms, alkyl groups, aryl groups, hydroxyl groups, alkoxy groups, phenol groups, and hydroxyl groups forming acids, R 2 and R 3 Can be the same or different.
[0045] The method for synthesizing deuterated co...
Embodiment 1
[0058] Embodiment 1: the synthesis of compound 2a
[0059]
[0060] a), iodo compound 1a (152mg), deuterated calcium hypophosphite (Ca(D 2 PO 2 ) 2 ,69.6mg, 0.8equiv), sodium bicarbonate (NaHCO 3 , 84.0mg, 2.0equiv), initiator azobisisobutylamidine hydrochloride (AIBA, 27mg, 0.2equiv) were weighed in 10mL reaction bottle, then add water (H 2 O, 2.0ml), react at 80 degrees Celsius. After the iodo compound 1a was consumed, the reaction was terminated (5h), and the solvent water was distilled off under reduced pressure. The obtained crude product was acetylated under the conditions of acetic anhydride and triethylamine, and after acylation, the crude product was extracted three times with ethyl acetate, the organic phases were combined, washed with saturated brine, washed with saturated sodium thiosulfate solution, and dried over anhydrous sodium sulfate. The crude product was concentrated to obtain compound 2a (142.0 mg) after separation and purification by column chroma...
Embodiment 2
[0071] Embodiment 2: the synthesis of compound 2b
[0072]
[0073] Iodo compound 1b (173.1mg), deuterated calcium hypophosphite (Ca(D 2 PO 2 ) 2 ,104.5mg, 1.2equiv), sodium bicarbonate (NaHCO 3 , 84.0mg, 2.0equiv), initiator azobisisobutylamidine dihydrochloride (AIBA, 27mg, 0.2equiv) were weighed in 10mL reaction flask, then add water and tert-butanol (H 2 O, 1.0ml of tert-butanol), reacted at 80 degrees Celsius, terminated the reaction (5h) after the iodide compound 1b was consumed, extracted the crude product with ethyl acetate 3 times, combined the organic phases, washed with saturated brine, and saturated thio Washed with sodium sulfate solution, dried over anhydrous sodium sulfate, concentrated to obtain a crude product, separated and purified by column chromatography to obtain compound 2b (102.4 mg), with a yield of 93% and a deuterated rate of 99%.
[0074] Compound 2b is a colorless oily substance.
[0075] 1 H NMR (600MHz, CDCl 3)δ7.39–7.25 (m, 5H), 4.78 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com