Multi-active-group red dye with high fixation rate and high chlorine fastness for printing and preparation method thereof
A technology of chlorine fastness and reactive dyes, applied in reactive dyes, dyeing methods, azo dyes, etc., can solve the problems of reactive chlorine oxidative damage, poor stability of chlorine resistance, discoloration, etc., to increase stability and improve resistance. Chlorine performance, the effect of improving the ability to resist effective chlorine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
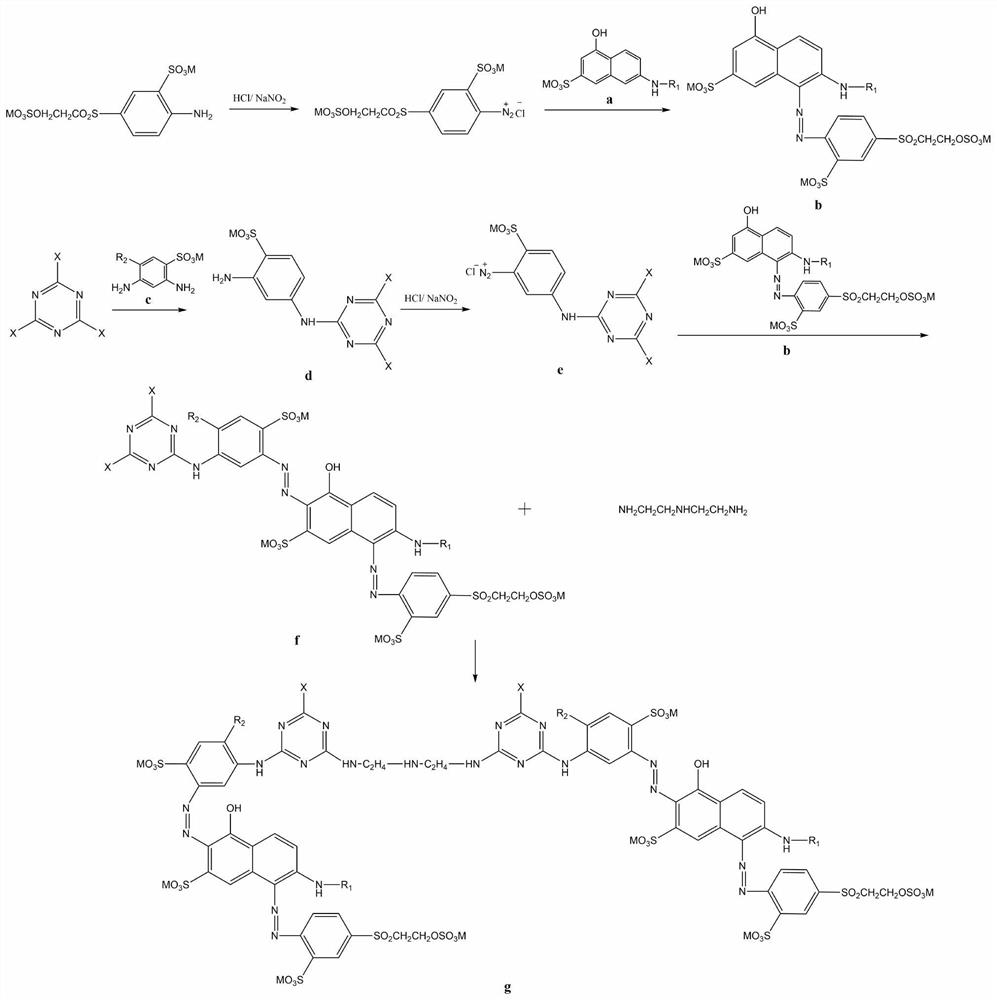
[0041] An embodiment provided by the present invention: a multi-reactive red dye with high color fixation rate and high chlorine fastness for printing, the reactive red dye is a compound shown in general structural formula I-1;
[0042]
[0043] The preparation method of the compound shown in the above-mentioned general formula I-1 is carried out according to the following process, and the specific steps include:
[0044] (1) Preparation of J-acid monoazo orange reactive dyes
[0045] A1: Add 36.1 g of 100% 4-β-ethylsulfone sulfate aniline-2-sulfonic acid, 200 g of ice and 20 mL of water into a 1000 mL beaker, and ice grind for 2 hours. Add 12.2 g of industrial hydrochloric acid (30%) and continue stirring for 1 hour. Dissolve 7.1g of 100% solid sodium nitrite in 30mL of water, and slowly add it dropwise to the system. During the dropwise addition, keep the reaction solution so that the Congo red test paper is slightly blue, and the KI test paper is slightly blue. After t...
Embodiment 2
[0056] An embodiment provided by the invention: a multi-reactive red dye with high color fixation rate and high chlorine fastness for printing, the reactive red dye is a compound shown in general structural formula I-2;
[0057]
[0058] In the present embodiment, the preparation method of reactive red dye I-2 and the beating device in the B1 step in the preparation method of reactive red dye are the same as in Example 1, the difference is that the difference is that N-methyl J acid replaces I-1 The J acid in the preparation step (1) prepares the corresponding reactive red dye, and the reactive red dye I-2 replaces the reactive dye I-1 to prepare the corresponding commercial dye.
Embodiment 3
[0060] An embodiment provided by the invention: a kind of multi-reactive red dye with high color fixation rate and high chlorine fastness for printing, the reactive red dye is a compound shown in general structural formula I-3:
[0061]
[0062] In the present embodiment, beating device is the same as embodiment 1 in the preparation method of reactive red dye I-3 and B1 step in the preparation method of reactive red dye, difference is that N-sulfomethyl J acid replaces I-1 preparation step ( 1) The J acid in 1) prepares the corresponding reactive red dye, and the reactive red dye I-3 replaces the reactive red dye I-1 to prepare the corresponding commercial dye.
PUM
| Property | Measurement | Unit |
|---|---|---|
| color fastness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com