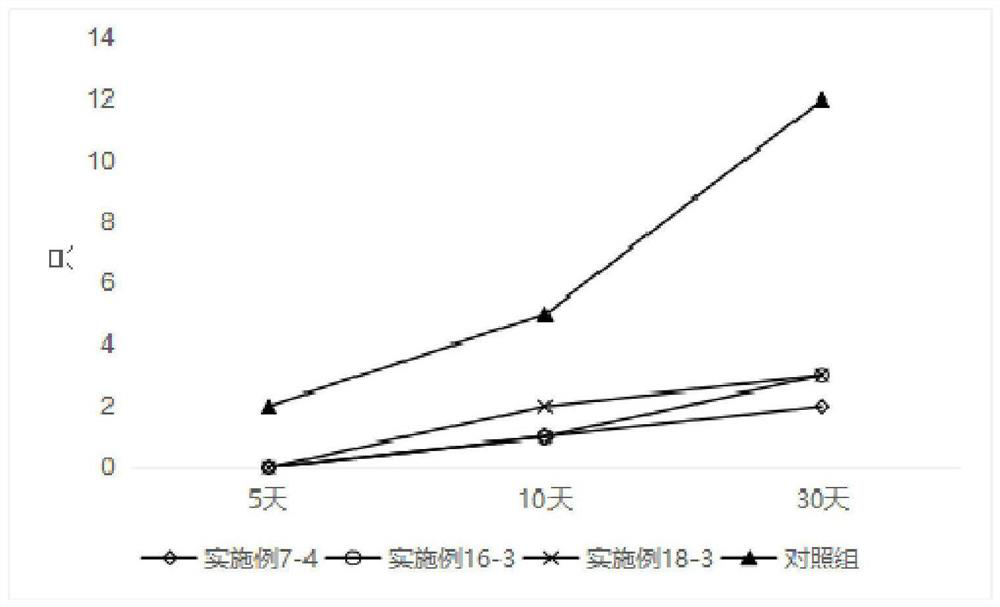
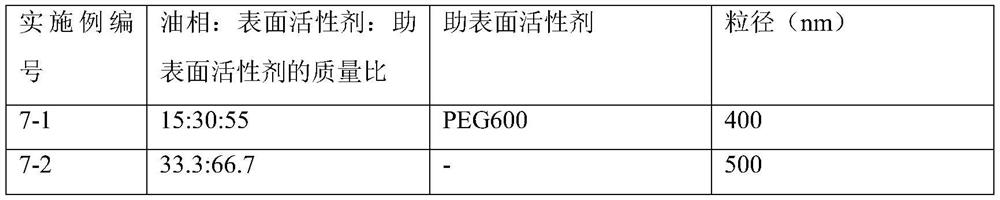
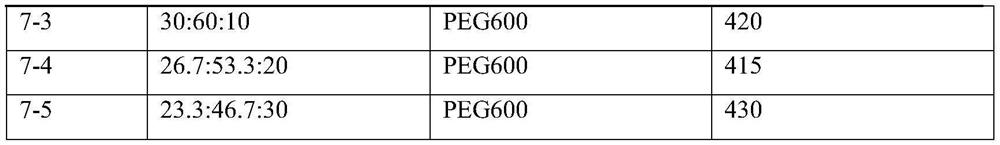
Self-microemulsion preparation of axitinib
A technology of axitinib and self-microemulsion, applied in the field of medicine, can solve problems such as difficult mixing, excessive uniformity, and high incidence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The prescription is as follows: Axitinib: 5mg;
[0051] The total mass of the SMEDDS carrier is 600mg: oil phase: surfactant: the mass ratio of co-surfactant is: 16.7:53.3:30; the oil phase is ethyl oleate; the surfactant is polyoxyethylene castor oil ; The co-surfactant is diethylene glycol monoethyl ether. The preparation process refers to Experiment 1, and the particle size measurement refers to Experiment 2. The particle diameter of the nanoemulsion of the obtained axitinib preparation is 18.45nm.
Embodiment 2
[0053] The prescription is as follows: Axitinib: 5mg;
[0054] SMEDDS carrier total mass is 600mg: oily phase: the mass ratio of surfactant is: 1:9; Described oily phase is glycerol monolinoleate; Described surfactant is caprylic capric acid polyethylene glycol glyceride and For the mixed emulsifier of polyoxyethylene castor oil, the mass ratio of the two is 3:1. The preparation process refers to Experiment 1, and the particle size measurement refers to Experiment 2. The particle diameter of the nanoemulsion of the obtained axitinib preparation is 114.54nm.
Embodiment 3
[0056] The prescription is as follows: Axitinib: 5mg;
[0057] The total mass of the SMEDDS carrier is 600mg: oil phase: surfactant: the mass ratio of co-surfactant is: 1:8:1; the oil phase is medium chain triglyceride; A mixed emulsifier of ethylene glycol glyceride and polyoxyethylene castor oil, the mass ratio of the two is 3:1; the co-surfactant is PEG400. The preparation process refers to Experiment 1, and the particle size measurement refers to Experiment 2. The particle diameter of the nanoemulsion of the obtained axitinib preparation is 170.45nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com