Cervical cancer therapeutic vaccine based on recombinant attenuated Listeria monocytogenes and preparation method of cervical cancer therapeutic vaccine
A therapeutic vaccine, Listeria monocytogenes technology, applied in the field of genetic engineering, can solve the problems of long immunization cycle and poor immunization effect, and achieve the effect of not easy to lose, no risk of antibiotic resistance, and efficient tumor killing mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] Still further, the embodiments of the present invention also provide a method for preparing the above-mentioned recombinant attenuated Listeria monocytogenes vaccine, comprising the following steps:
[0053] (1) Synthesize the complete open reading frame (ORF) sequence of the HPV 16 type E7 gene;
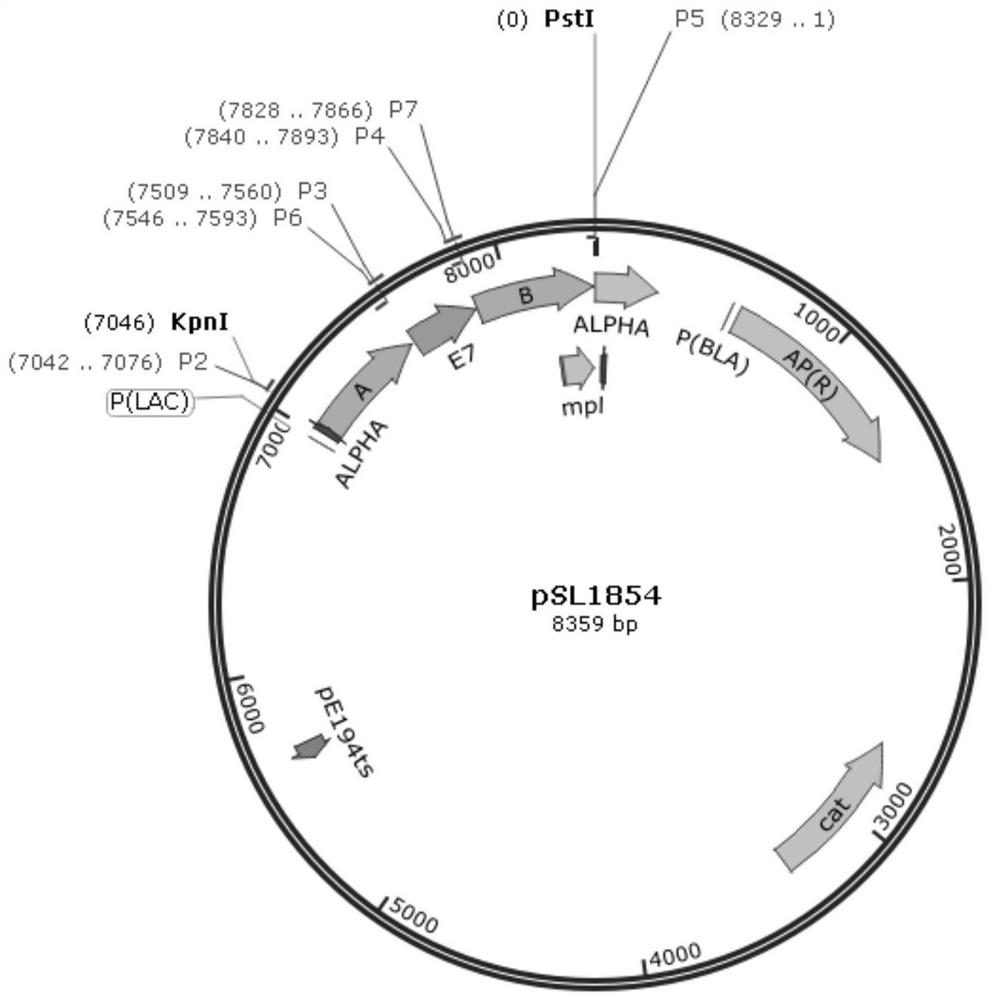
[0054] (2) Construction of the homologous recombination plasmid pSL1854 used in the present invention;
[0055] (3) preparing attenuated and single-increased Listeria d competent cells;
[0056] (4) using the recombinant plasmid prepared in step (2) to perform electrotransformation on the competent cells prepared in step (3);
[0057] (5) Screening of the vaccine (LADS-Echo7) of the present invention.
[0058] In one embodiment, the step (1) specifically includes:
[0059] Download the ORF sequence of HPV type 16 E7 gene from NCBI, the length is 297bp, and entrust Suzhou Jinweizhi Biotechnology Company to synthesize the sequence.
[0060] In one embodiment, the step (2) s...
Embodiment 1
[0076] Example 1 Construction of recombinant Listeria vaccine LADS-Echo7
[0077] 1. Synthesis of full-length gene sequence of HPV type 16 E7 and construction of homologous recombination plasmid
[0078] Download the complete open reading frame (ORF) sequence (as shown in SEQ ID NO.1) of the HPV type 16 E7 gene from the Genbank database, and entrust Suzhou Jinweizhi Biotechnology Company to synthesize the sequence. PCR was used to amplify fragments A (498bp, amplification primers P2 and P3) and B (500bp, amplification primers P4 and P5) respectively containing restriction sites KpnI and PstI using the whole genome DNA of Lemo-C07 as a template; At the same time, fragment E7 was amplified using the complete sequence of the synthesized E7 gene as a template (amplification primers were P6 and P7). On this basis, the three fragments were joined together by overlapping PCR technique (SOE-PCR) to obtain the target fragment of "A-E7-B" (sequence shown in SEQ ID NO.2). Using KpnI an...
Embodiment 2
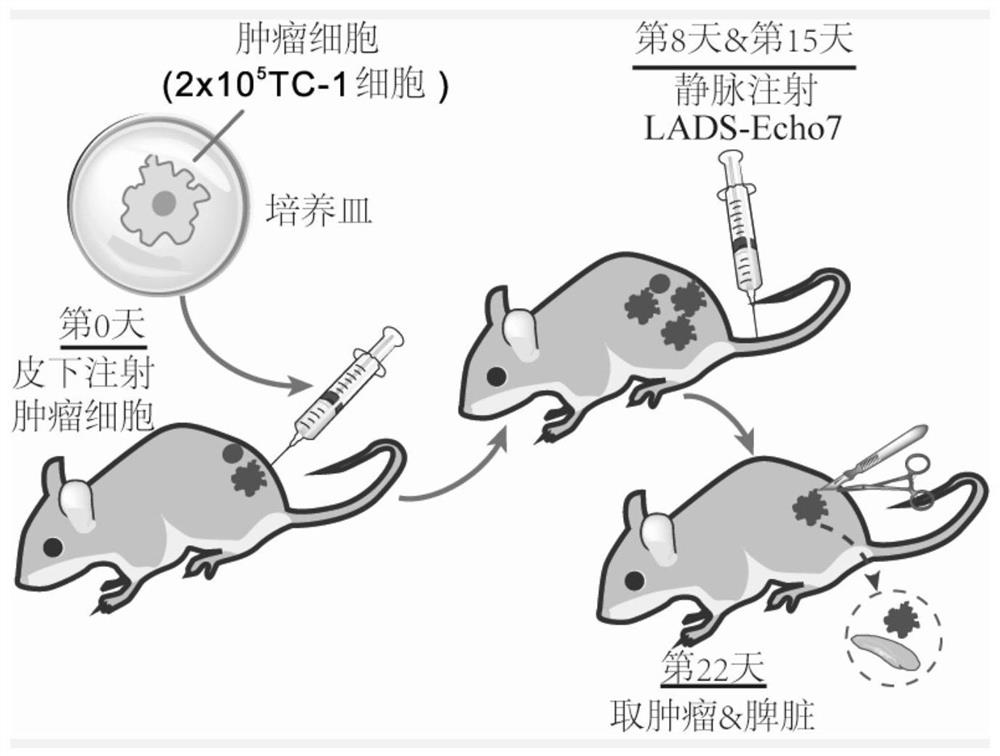
[0089] Example 2 Efficacy Evaluation of Recombinant Listeria Vaccine LADS-Echo7 Treating Mouse Model of Cervical Cancer
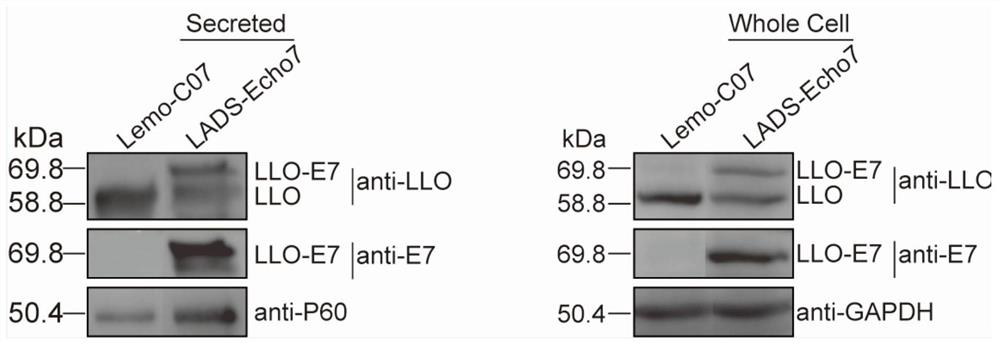
[0090] 1. Verification of expression of recombinant tumor antigen E7 protein by LADS-Echo7
[0091] Pick a single colony in 5mL BHI liquid medium and place it on a shaker at 37°C for overnight culture. Take 1 mL of the overnight culture and transfer it to 100 mL of BHI liquid medium, shake and culture at 37°C for 8-9 hours, centrifuge and filter the supernatant; precipitate the filtered supernatant with trichloroacetic acid overnight, 12000rpm, 20min, 4 Centrifuge at ℃ to discard the supernatant, resuspend the pellet with 1mL 1M NaOH, centrifuge to take the supernatant, which is the secreted protein; after centrifugation, the pellet is washed with 50mM PBS and resuspended with 1mL Lister lysate, broken by a homogenizer, and centrifuged to take the supernatant. Qing is a cytoplasmic protein. After measuring and quantifying the secreted protein and cytoplas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com