Near-infrared silicon-based rhodamine fluorescent dye, preparation method and application of near-infrared silicon-based rhodamine fluorescent dye in mitochondrial meninges in-situ no-clean imaging
A silicon-based rhodamine and fluorescent dye technology, applied in the field of near-infrared fluorescent dyes, can solve the problems of weak resistance to photobleaching, difficult to locate mitochondrial inner membrane, and unsuitable for long-term continuous imaging.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] The synthetic route of near-infrared silicon-based rhodamine dye SiR is as follows:
[0093]
[0094] Synthesis of compounds 1-4
[0095] 3-Bromo-N,N-dimethylaniline (5.00g, 25mmol) was dissolved in 50mL of acetic acid, 37% formaldehyde solution (7mL, 11mmol) was added, and reacted at 60°C for 30min. After cooling, evaporate the acetic acid, add NaHCO 3The saturated solution was adjusted to neutral pH, extracted with dichloromethane, the solvent was distilled off, and separated by silica gel column chromatography to obtain intermediate 1-2. In a three-necked flask, the obtained intermediate 1-2 was dissolved in anhydrous tetrahydrofuran, and under nitrogen protection and -78°C, 1.3M sec-butyllithium (14mL, 18.8mmol) was slowly added dropwise, reacted at low temperature for 20min, and then slowly A tetrahydrofuran solution of dimethyldichlorosilane (2.94 g, 22.72 mmol) was added dropwise, and the reaction was continued for 2 h. Quench the reaction with 2M HCl aqu...
Embodiment 2
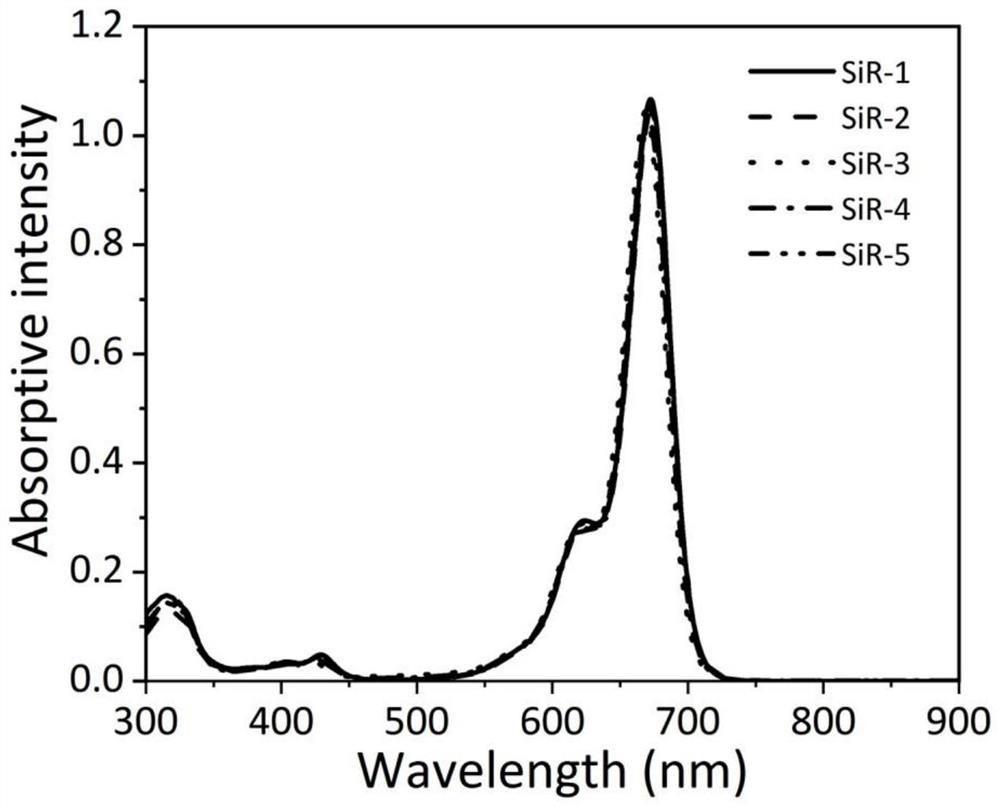
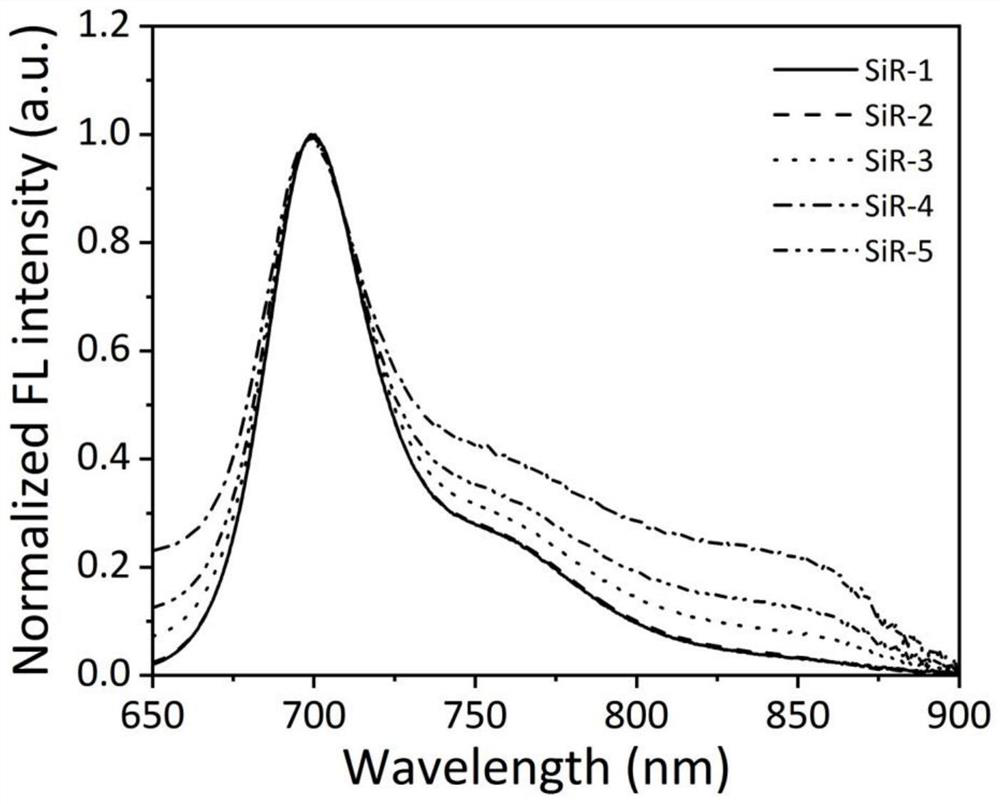
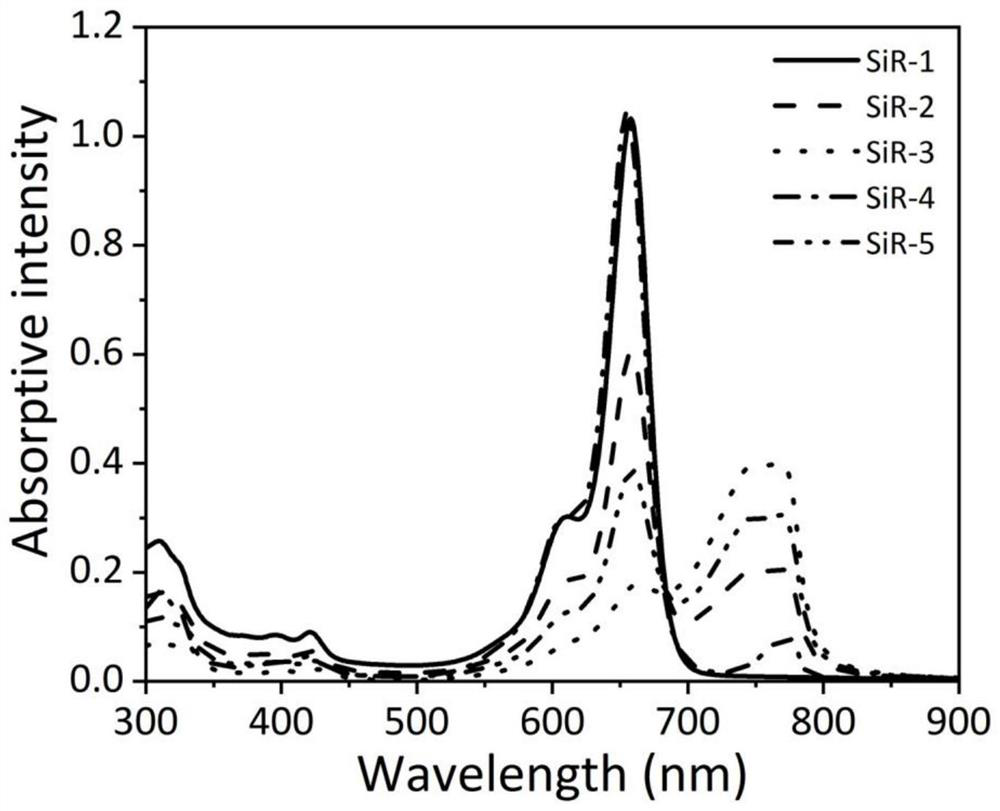
[0123] Using silicon-based rhodamine dye SiR as a fluorescent imaging reagent, its spectral performance in different solutions was tested.
[0124] (1) Preparation of stock solution
[0125] Preparation of silicon-based rhodamine dye SiR stock solution: Accurately weigh five SiR dyes and dissolve them in DMSO to prepare a concentration of 10×10 -3 The mother liquor of M was stored in a -20°C refrigerator for later use.
[0126] Preparation of lipid film solution: accurately weigh 32.3mg dimyristoylphosphatidylcholine (DMPC) and 8.21mg sodium dimyristoylphosphatidylglycerophosphate (DMPG) and dissolve in 20mL dichloromethane-methanol solution [V (di Chloromethane) / V (methanol)=4 / 1]. Evaporate the solvent under reduced pressure to form a lipid film, dry in vacuum for 2 hours to remove the residual solvent, then add 28.9 mL of sodium chloride (100 mM) solution to dissolve it completely, then pass argon gas for 10 minutes to remove the dissolved oxygen in the solution, and ultra...
Embodiment 3
[0133] Toxicity test of silicon-based rhodamine dye SiR-3 on HeLa cells
[0134] (1) Cell culture
[0135] Human cervical cancer cell line HeLa cells were cultured in high-glucose DMEM medium containing 10% newborn calf serum in 5% CO 2 , cultured in a 37°C incubator with a humidity of 80%.
[0137] When the cells grow to a confluence of about 90%, discard the old culture medium, rinse twice with phosphate buffer, add an appropriate amount of 0.25% trypsin to digest, pay attention to observation during the period, and add 1-2mL immediately when the cells fall off in pieces Fresh medium was used to stop the digestion, and the cell suspension was transferred to a 15mL centrifuge tube, centrifuged at room temperature at 700 rpm for 5min, and the supernatant was discarded. Resuspend the cells with 1 mL of the corresponding culture medium.
[0138] (3) Cell count
[0139] Take 10 μL of the cell suspension into a 0.50 mL centrifuge tube, add 10 μL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com