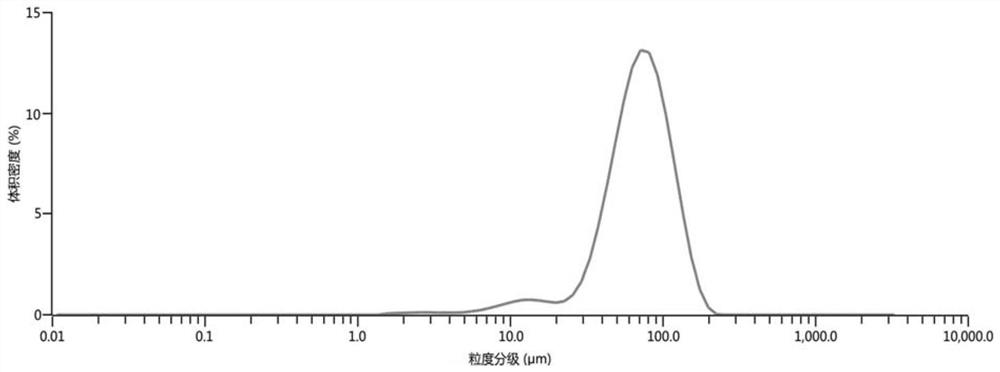
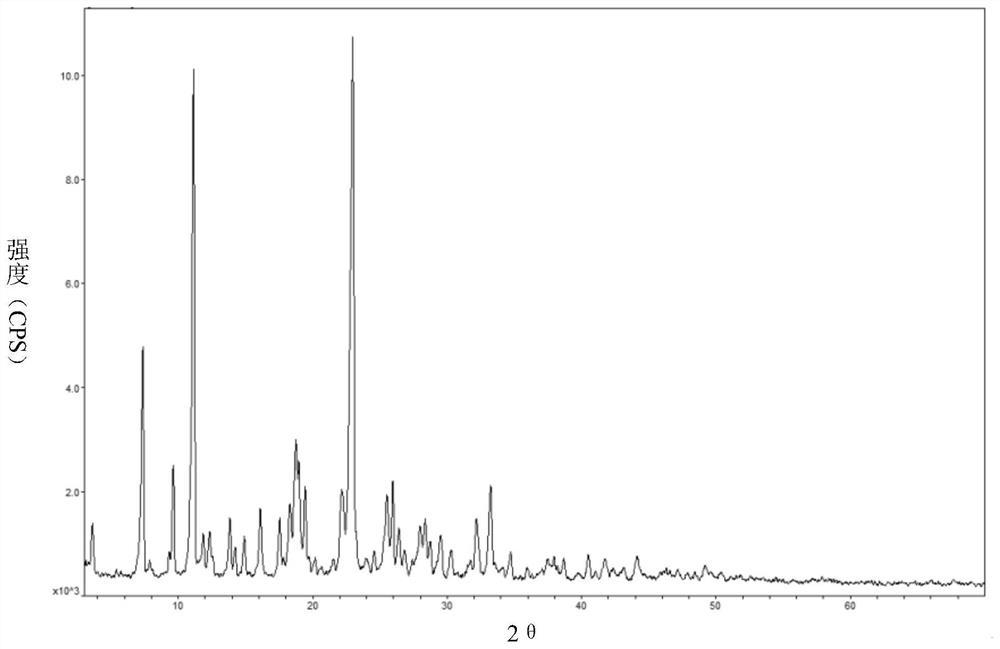
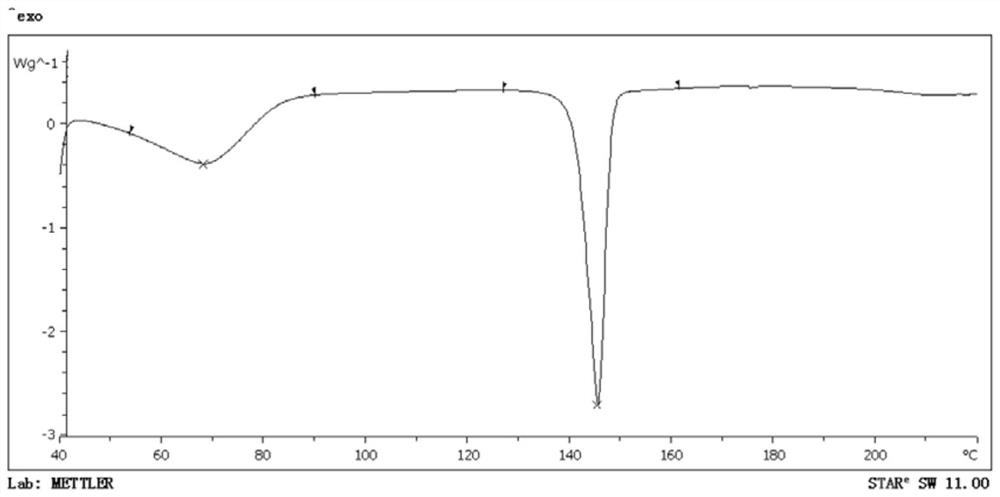
Amlodipine mesylate monohydrate and its preparation method and use
A technology of amlodipine mesylate and monohydrate, which is applied in the field of amlodipine mesylate monohydrate and its preparation, can solve the problems of unsatisfactory particle size and uniformity, fluidity and compressibility, etc. Achieve uniform and controllable particle size distribution, good content uniformity, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0080] Experimental example 1 amlodipine free base is that raw material is that starting material prepares amlodipine mesylate monohydrate
[0081] Add 100 g of amlodipine free base (245.1 mmol), ethyl acetate (500 mL) and purified water (5.29 mL, 1.2 equivalents) to a 2000 mL three-necked flask in sequence, and stir for 15 minutes to dissolve. Under room temperature conditions, first weigh 5.88g methanesulfonic acid (61.3mmol), quickly add in the reaction solution through a constant pressure dropping funnel under stirring, control the dropping rate to be 5mL / min, and control the stirring speed at 160 rpm to obtain A clear solution. At room temperature, 1.25 g of amlodipine mesylate monohydrate seed crystals were added to the solution to obtain a suspension, and the stirring speed was controlled at 70 rpm. Separately take by weighing 17.6g methanesulfonic acid (183.9mmol), carry out slowly dropwise under stirring by constant pressure dropping funnel, rate of addition is 0.5...
experiment example 2
[0083] Experimental Example 2 Amlodipine free base is the raw material for the preparation of amlodipine mesylate monohydrate as the starting material
[0084] Add 100 g of amlodipine free base (245.1 mmol), ethyl acetate (500 mL) and purified water (5.29 mL, 1.2 equivalents) to a 2000 mL three-necked flask in sequence, and stir for 15 minutes to dissolve. At room temperature (25°C), first weigh 5.88g methanesulfonic acid (61.3mmol), quickly add it into the reaction solution through a constant pressure dropping funnel under stirring, control the dropping rate to 5mL / min, and control the stirring speed at 160 rpm / min to obtain a clear solution. At room temperature, 1.25 g of amlodipine mesylate monohydrate seed crystals were added to the solution to obtain a suspension, and the stirring speed was controlled at 70 rpm. Take by weighing 17.6g methanesulfonic acid (183.9mmol) separately, carry out slowly dropwise under stirring by constant pressure dropping funnel, rate of add...
experiment example 3
[0085] Experimental Example 3 Amlodipine free base is the raw material for the preparation of amlodipine mesylate monohydrate as the starting material
[0086] Add 210g of amlodipine free base (514.7mmol), ethyl acetate (1000mL) and purified water (11.1mL, 1.2 equivalents) to a 3000mL three-necked flask in sequence, and stir for 15 minutes to dissolve. Under low temperature conditions (5°C), first weigh 12.3g methanesulfonic acid (128.7mmol), quickly add it into the reaction solution through a constant pressure dropping funnel under stirring, control the dropping rate to 5mL / min, and control the stirring speed at 160 rpm / min to obtain a clear solution. At room temperature, 2.6 g of amlodipine mesylate monohydrate seed crystals were added to the solution to obtain a suspension, and the stirring speed was controlled at 270 rpm. Another 37.1g methanesulfonic acid (386.0mmol) was weighed, slowly added dropwise with stirring through a constant pressure dropping funnel, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com