Preparation method of dalbavancin key intermediate A40926
A technology of A40926 and dalbavancin, which is applied in the field of preparation of multi-component A40926, a key intermediate of dalbavancin, can solve the problems of cumbersome operations, complicated washing and analytical operations, unfavorable industrial production and the like, and achieves simple unit operation, The effect of short process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
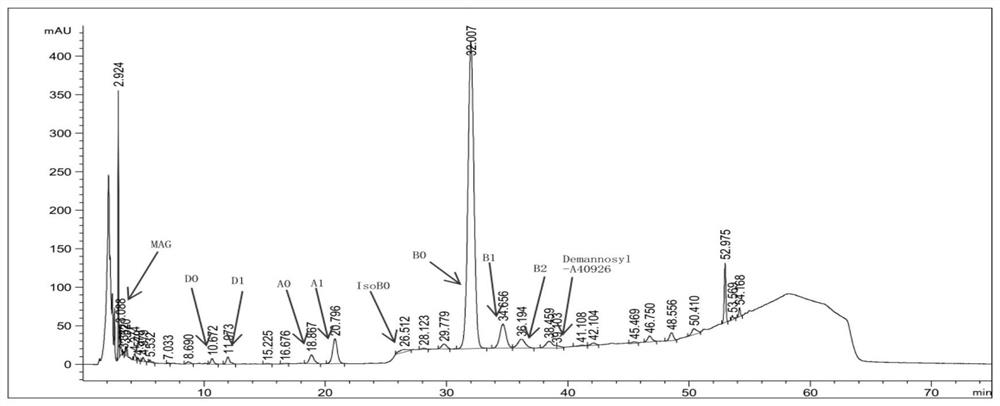
[0041] A40926 fermentation broth 10000L, A40926-B0 unit is 1880ug / mL, adjust the pH to 11.2 with 4% sodium hydroxide solution, control the temperature of the material at about 25°C, and stir for deacylation. After stirring for 2 hours, samples were taken to detect the A40926 content and the residual amounts of component precursor substances PA and PB; the test results were as follows: figure 2 and shown in Table 1. As can be seen from Table 1, the HPLC purity of the effective components of A40926 is 78.09%, and the alkali degradation impurity IsoB0 is 0.84%, which is effectively controlled within the limit. figure 2 It shows that there are no residues of PA and PB, indicating that the deacylation has been completed. Then add 2% perlite (to improve the filtration efficiency), plate and frame filter press, 2h press filter is completed to obtain deacylation filtrate, top wash 2500L drinking water, combine deacylation filtrate and top wash, a total of 9500L. The deacylation fi...
Embodiment 2
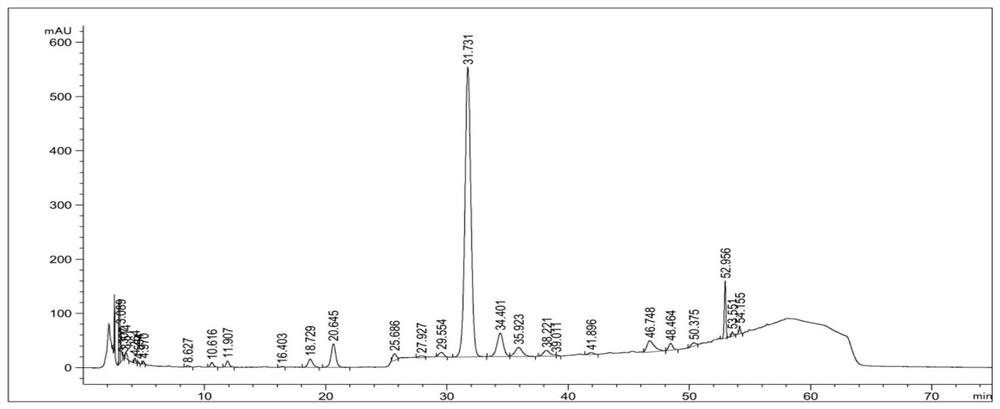
[0045] The 9500L combined solution obtained by combining the deacylation filtrate and the top washing solution in Example 1 was passed into 1000L macroporous resin HZ-818 (resin particle diameter is 0.3 ~ 1.2mm), and the loading amount was 17.20g A40926-B0 / L Resin, the flow rate is 500-1500L / h, the color of the effluent is dark and solids are precipitated, after the sample is loaded, wash 4000L0.1mol / L hydrochloric acid aqueous solution, the effluent is light yellow liquid, showing acidity, analyze 2500L 70% ethanol solution ( The aqueous phase contains 0.3mol / L hydrochloric acid), and the analysis solution above 500ug / mL of A40926-B0 is combined to obtain 1500L analysis combined solution ①, and the HPLC detection results are as follows image 3 As shown in Table 2, the effective components are well retained, and the impurities in the HPLC chromatogram are clearly removed in 0 to 5 minutes. The analysis combined solution ① was a brown transparent liquid, and the A40926-B0 unit...
Embodiment 3
[0049] Get the analysis combined liquid of embodiment 2 1. 150L, add water and dilute to 600L, add 6kg sodium chloride (1% sodium chloride is added in the chromatography sample liquid), adjust the pH8. 5,1m 2 Plate and frame filtration, the filtrate is passed into 100L UniPMM40-500 packing at a flow rate of 200-400L / h for sample loading, the sample loading is 16.56g A40926-B0 / L packing, and 900L pH10.7 buffered salt solution is analyzed at a flow rate of 300-500L / h (0.02mol / L sodium bicarbonate solution, adjust pH with 20% sodium hydroxide solution), get the analysis solution and carry out HPLC detection, after HPLC detection, merge analysis solution 520L, HPLC detection result is as follows Figure 4 As shown in Table 3, that is, the analytical combined solution ②, its A40926-B0 unit is 2880ug / mL, which is a light yellow transparent liquid. After chromatography, the effective components of the target product are well retained, and the HPLC purity is greatly improved. , Iso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com