Quality control method of rhizoma wenyujin concisum formula granules
A quality control method and technology of formulated granules, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of low polarity of gemmaconone, questionable quality index, poor water solubility, etc., to achieve improved peak shape, high detection efficiency, Accurate effect of quality judgment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
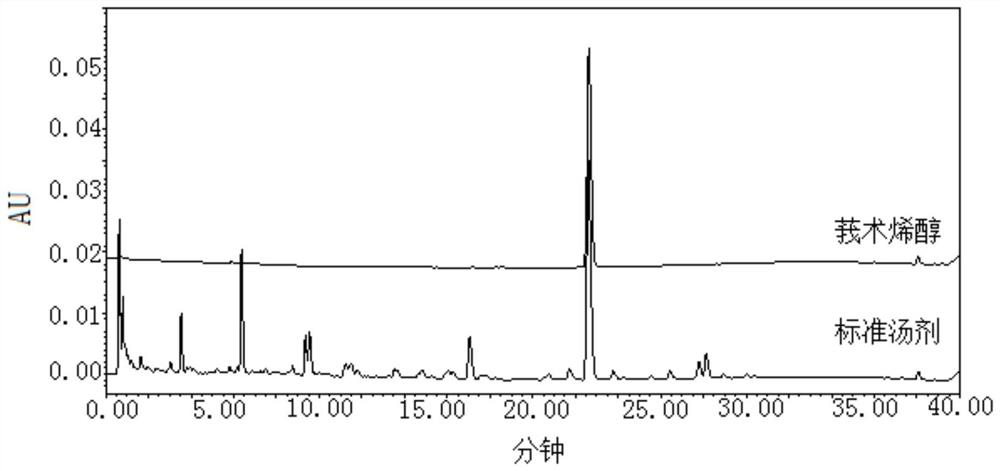
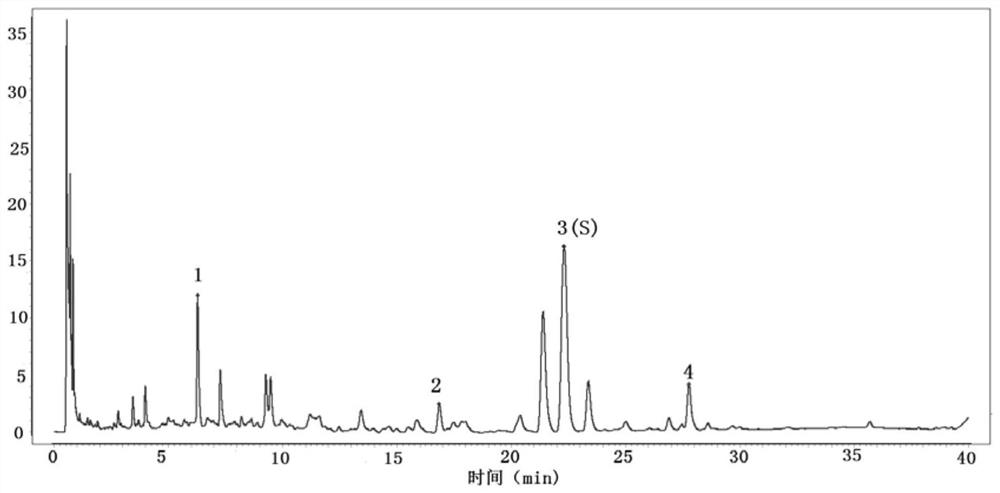
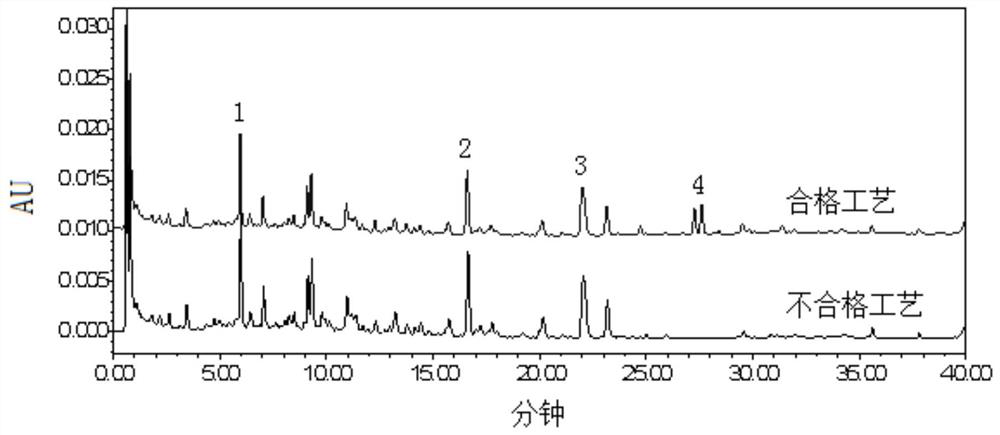
[0034] A quality control method for sliced turmeric formula granules, comprising adopting high performance liquid chromatography to obtain the characteristic spectrum of the test solution, in which the chromatographic peak of curcumenol is used as the reference peak S, and the relationship between each characteristic peak and the reference peak S is calculated. The relative retention time of peak S, wherein the relative retention time of at least 3 characteristic peaks is within ±10% of the specified value, and the specified value is: 0.28, 0.76, 1.25.
[0035] The chromatographic conditions of the high performance liquid chromatography in this embodiment are: using octadecylsilane bonded silica gel as filler, Waters BEH C18 chromatographic column, the column length of the chromatographic column is 100mm, the inner diameter of the column is 2.1mm, the particle diameter with acetonitrile as mobile phase A and 0.1% phosphoric acid as mobile phase B, carry out gradient elution a...
Embodiment 2
[0063] In this embodiment, conventional methods are used to verify the precision, repeatability and stability of the characteristic map, as follows:
[0064] Take 6 parts of the test solution, obtain its characteristic spectrum, take the curcumenol peak as the reference peak, calculate its relative peak area and relative retention time. And calculate the RSD. Results The RSD of the relative retention time of each characteristic peak was in the range of 0.0%-0.1%, and the RSD of the relative peak area was in the range of 0.2%-2.4%, which indicated that the repeatability of the characteristic spectrum was good.
[0065] Using Waters UPLC H-Class, TUV detector, take the test solution, obtain its characteristic spectrum, take the curcumenol peak as the reference peak, calculate its relative peak area and relative retention time. And calculate the RSD. Results In the characteristic spectrum obtained by Waters UPLCH-Class and TUV detector, the relative retention time of each chara...
Embodiment 3
[0068] This embodiment provides the quality control method of the turmeric formula granules, which, in addition to the quality control of the characteristic spectrum recorded in Example 1, also includes the quantitative control of the quality evaluation index components in the turmeric formula granules, including the control of curcumenol The content in turmeric formula granules is not less than 4.5mg / g.
[0069] The chromatographic conditions used in this example are basically the same as those in Example 1, the only difference being that the detection wavelength is adjusted to 260nm for detection.
[0070] Using the chromatographic conditions of this example, first draw the standard curve of curcumenol, and obtain the regression equation of curcumenol as y=4542.0025x+1445.5373, R=1.0000, and its linear range is 6.844 μg / ml~219.010 μg / ml.
[0071] Then adopt the detection method of the present embodiment to carry out the detection of 20 batches of turmeric standard decoctio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com