Amantadine hydrochloride impurity removal method
A technology for amantadine hydrochloride and amantadine, which is applied in the preparation of amino compounds from amines, purification/separation of amino compounds, and organic chemistry, etc. Synthesis process, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
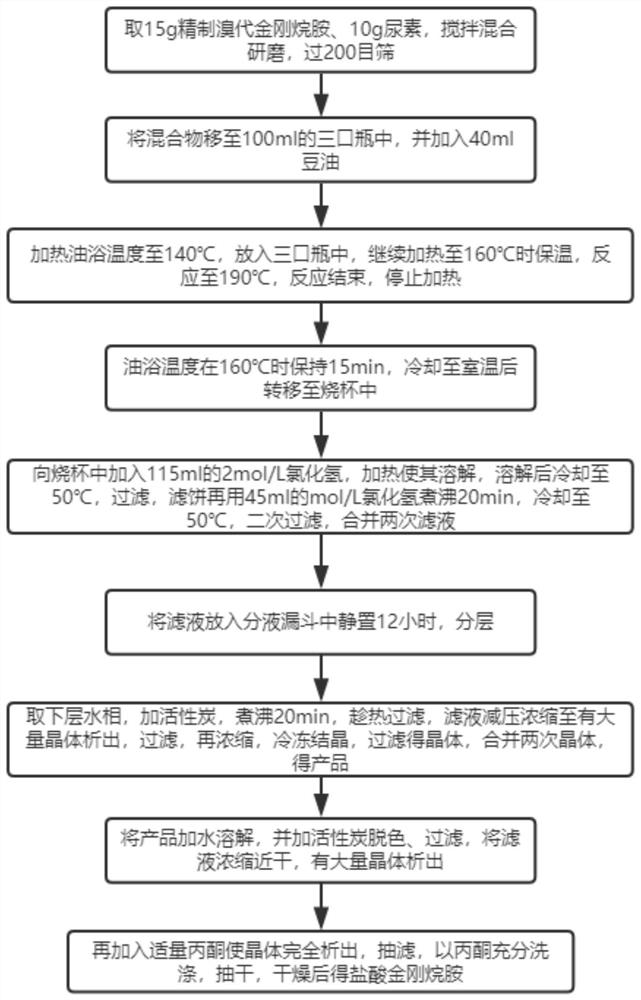
[0036] A method for removing impurities of amantadine hydrochloride, such as figure 1 shown, including the following steps:
[0037] S1: Take 15g of refined amantadine bromide and 10g of urea, stir, mix and grind, and pass through a 200-mesh sieve;
[0038] S2: Move the mixture to a 100ml three-necked bottle, and add 40ml of soybean oil;
[0039] S3: Heat the temperature of the oil bath to 140°C, put it into a three-neck bottle, continue heating to 160°C, keep it warm, react to 190°C, stop the heating when the reaction is over;
[0040] S4: Keep the temperature of the oil bath at 160°C for 15 minutes, transfer to a beaker after cooling to room temperature;
[0041] S5: Add 115ml of 2mol / L hydrogen chloride into the beaker, heat to dissolve, cool to 50°C after dissolving, filter, boil the filter cake with 45ml of mol / L hydrogen chloride for 20min, cool to 50°C, filter twice, and combine twice the filtrate;
[0042] S6: Put the filtrate into a separatory funnel and let it st...
Embodiment 2
[0058] A method for removing impurities of amantadine hydrochloride, such as figure 1 shown, including the following steps:
[0059] S1: Take 15g of refined amantadine bromide and 10g of urea, stir, mix and grind, and pass through a 200-mesh sieve;
[0060] S2: Move the mixture to a 100ml three-necked bottle, and add 40ml of soybean oil;
[0061] S3: Heat the temperature of the oil bath to 140°C, put it into a three-neck bottle, continue heating to 160°C, keep it warm, react to 190°C, stop the heating when the reaction is over;
[0062] S4: Keep the temperature of the oil bath at 160°C for 15 minutes, transfer to a beaker after cooling to room temperature;
[0063] S5: Add 115ml of 2mol / L hydrogen chloride to the beaker, heat to dissolve, cool to 50°C after dissolving, filter, boil the filter cake with 45ml of mol / L hydrogen chloride for 20min, cool to 50°C, filter twice, and combine twice the filtrate;
[0064] S6: Put the filtrate into a separatory funnel and let it stan...
Embodiment 3
[0079] A method for removing impurities of amantadine hydrochloride, such as figure 1 shown, including the following steps:
[0080] S1: Take 15g of refined amantadine bromide and 10g of urea, stir, mix and grind, and pass through a 200-mesh sieve;
[0081] S2: Move the mixture to a 100ml three-necked bottle, and add 40ml of soybean oil;
[0082] S3: Heat the temperature of the oil bath to 140°C, put it into a three-neck bottle, continue heating to 160°C, keep it warm, react to 190°C, stop the heating when the reaction is over;
[0083] S4: Keep the temperature of the oil bath at 160°C for 15 minutes, transfer to a beaker after cooling to room temperature;
[0084] S5: Add 115ml of 2mol / L hydrogen chloride to the beaker, heat to dissolve, cool to 50°C after dissolving, filter, boil the filter cake with 45ml of mol / L hydrogen chloride for 20min, cool to 50°C, filter twice, and combine twice the filtrate;
[0085] S6: Put the filtrate into a separatory funnel and let it stan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com