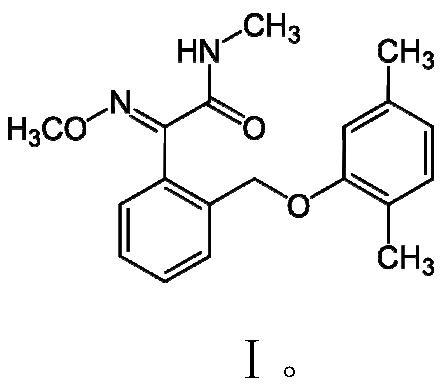
Preparation method of dimoxystrobin
A technology of kresastrobin and methoxyamine salt, which is applied in the field of pesticide chemistry, can solve the problems of many side reactions, high price, cumbersome operation, etc., and achieve the goal of reducing double grignard side reactions, eliminating side reactions, and easy access to raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
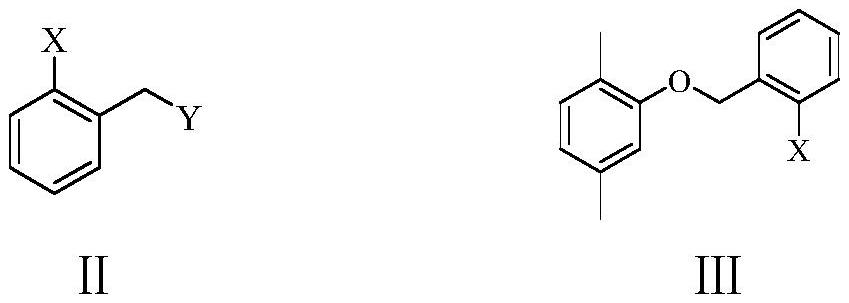
[0071] Example 1: 2-(2,5-dimethylphenoxymethyl) bromobenzene (Ⅲ 1 ) preparation
[0072] To a 1000 ml four-neck flask connected with stirring, a thermometer, a constant pressure dropping funnel and a reflux condenser, add 400 g of 1,2-dichloroethane, 82.8 g (0.6 mol) of potassium carbonate, 122.2 g (1.0 mol) ) 2,5-dimethylphenol, heated, kept between 40 and 45°C, added dropwise 215.8 grams (1.05 moles) of 2-chloromethylbromobenzene (Ⅱ 1 ), the dropwise addition was completed in about 3 hours, after which the reaction was stirred at 45 to 50°C for 2 hours, cooled to 20-25°C, filtered, the filter cake was washed with 1,2-dichloroethane, 100 grams each time, and the organic phases were combined, Distillation recovery solvent, vacuum distillation (150-175 ° C / 1-2mmHg) to obtain 275.7 grams of 2-(2,5-dimethylphenoxymethyl) bromobenzene (Ⅲ 1 ), yield 94.6%, gas phase purity 99.9%.
[0073] The NMR data of the resulting product are as follows:
[0074] 1 H NMR (400MHz, DMSO-d ...
Embodiment 2
[0075] Example 2: 2-(2,5-dimethylphenoxymethyl) bromobenzene (Ⅲ 1 ) preparation
[0076] To a 1000 ml four-necked flask connected with stirring, a thermometer, a constant pressure dropping funnel and a reflux condenser, add 500 g of dichloromethane, 82.8 g (0.6 mol) of potassium carbonate, 122.2 g (1.0 mol) of 2,5- Dimethylphenol, heated, kept between 30 and 35 ° C, added dropwise 262.5 grams (1.05 moles) of 2-bromomethyl bromobenzene (II 2 ), the dropwise addition was completed in about 3 hours, thereafter 35 to 40°C stirred and reacted for 2 hours, cooled to 20-25°C, filtered, the filter cake was washed with dichloromethane, 100 grams each time, the organic phases were combined, the solvent was recovered by distillation, and the Pressure distillation (150-175 ℃ / 1-2mmHg) obtains 273.8 grams of 2-(2,5-dimethylphenoxymethyl) bromobenzene (Ⅲ 1 ), yield 94.0%, gas phase purity 99.8%.
Embodiment 3
[0077] Example 3: Preparation of N-methyl-2-(2,5-dimethylphenoxymethyl)phenyloxalamide (Ⅴ)
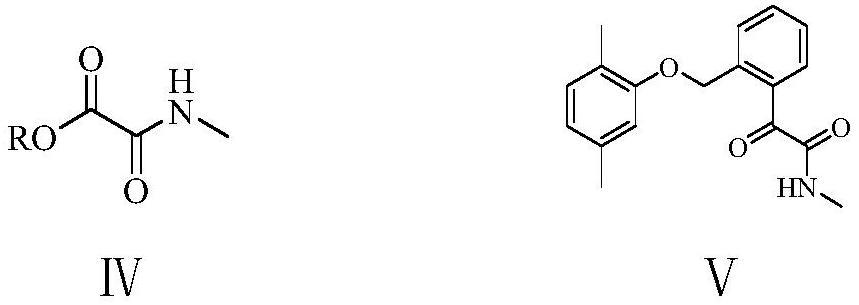
[0078] Under nitrogen protection, 150 grams of tetrahydrofuran, 2.7 grams (0.11 moles) of magnesium powder, 0.5 grams of 1,2-dibromoethane, 1.5 grams of 2 prepared in Example 2 were added to a 500 milliliter four-necked flask equipped with a stirring and thermometer. -(2,5-Dimethylphenoxymethyl)bromobenzene(Ⅲ 1 ), 0.05 gram of iodine, and initiated Grignard reaction at 40-45°C for 0.2 hours; between 40-45°C, 27.6 grams (0.1 moles in total) of 2-(2,5-dimethylphenoxy) prepared in Example 1 were added dropwise Methyl)bromobenzene(Ⅲ 1 ) and 100 grams of tetrahydrofuran, drop it in 2 hours, then stir and react at 45-50°C under nitrogen for 3 hours, and cool to 20-25°C to obtain a reaction solution containing Grignard reagent; Transfer the liquid to a constant pressure dropping funnel, keep it between 20-25°C, and add it dropwise to a mixed solution of 17.6 grams (0.15 moles) of N-methyl o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com