Methylquinolin-benzopyrylium derivative and preparation method and application thereof
A technology of benzopyrylium and methylquinoline, which is applied in the field of methylquinoline-benzopyrylium derivatives and their preparation, can solve the problems of poor sensitivity, application limitation, long response time, etc., and achieves enhanced Electrophilicity, achieve ultra-sensitive response, speed up effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
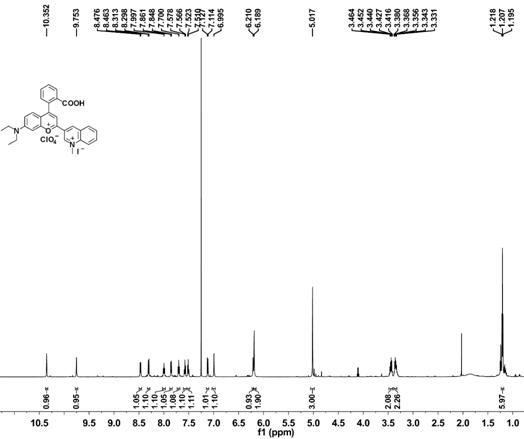
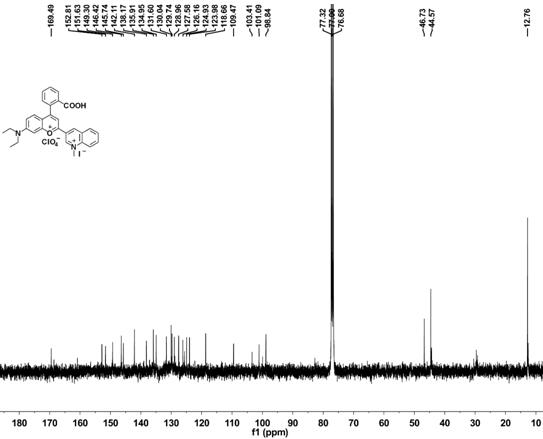
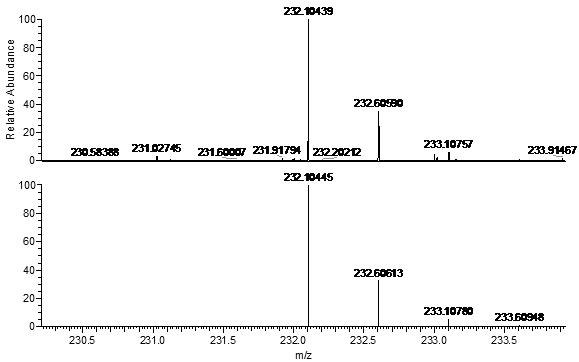
[0057] Embodiment 1 Synthesis of methylquinoline-benzopyrylium derivatives
[0058] (1) Synthesis of compound 1
[0059]
[0060] Put 3-diethylaminophenol (60.52 mmol, 10 g) and phthalic anhydride (60.52 mmol, 8.96 g) in a round bottom flask, add toluene (100 mL) under argon atmosphere, and reflux at 110 °C After reacting for 4 hours, cool the reaction mixture to 50-60°C, then add NaOH (50 mL) aqueous solution with a mass concentration of 35%, and react at 90°C for 6 hours. Acidified and placed at room temperature for 2 h, a precipitate precipitated out, filtered the precipitate, and recrystallized from ethanol to obtain a pale pink solid 2-(4-(diethylamino)-2-hydroxybenzoyl)benzoic acid (compound 1), the yield 89%.
[0061] (2) Synthesis of compound 2
[0062]
[0063] Compound (1 mmol, 313 mg) and 3-acetylquinoline (1 mmol, 171 mg) were dissolved in methanesulfonic acid (3 mL), reacted at 90 ° C for 9 h, the reaction system was cooled to room temperature, and Slowl...
Embodiment 2
[0070] Embodiment 2 CMQ is to SO in water phase system 2 selective detection of
[0071] Prepare CMQ with an initial concentration of 3 mM, prepare various anions and amino acids with an initial concentration of 30 mM, add 2.98 mL PBS and 10 μL CMQ to the fluorescence cuvette, and then add 10 μL sodium bisulfite (10 μM ), sodium sulfite (10 μM), sodium cyanide, sodium carbonate, sodium bicarbonate, sodium sulfate, sodium acetate, sodium nitrate, sodium chloride, sodium sulfide (10 μM), sodium sulfide (20 μM), sodium phosphate, bromine Sodium Chloride, Sodium Fluoride, Cysteine, Homocysteine, Glutathione, L-Serine, DL-Methionine, L-Phenylalanine, L-Lysine, L-Leucine, L-Proline, L-Histidine solution, SO only 2 Two forms of HSO exist in the aqueous system 3 - (sodium bisulfite) and SO 3 2- (Sodium sulfite) can significantly enhance the fluorescence intensity of CMQ at 640 nm, while other anions and amino acids cannot significantly change the fluorescence intensity of CMQ at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com