Truncated body of Seroin protein and application of truncated body
A protein and sequence technology, applied in the application field of the protein truncation body, can solve the problems of incomplete folding, high synthesis cost, host toxicity or growth inhibition, etc., to reduce cost and difficulty, low production cost, shorten molecular weight Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
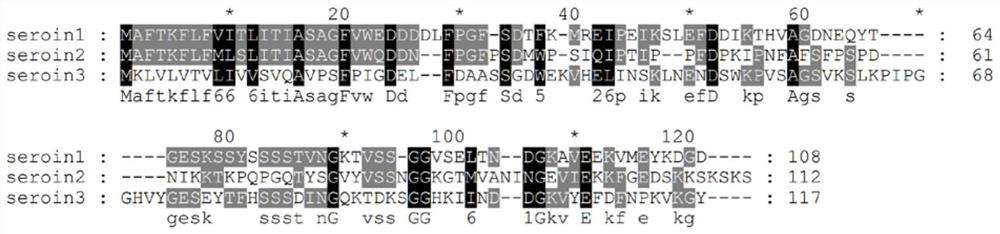
[0024] Embodiment 1, seroin protein bioinformatics analysis
[0025] Nucleosides of seroin1 (gene number gi|19070653), seroin2 (gene number gi|19070655) and seroin3 (gene number gi|512931752) downloaded from the NCBI database (https: / / www.ncbi.nlm.nih.gov / ) The amino acid and amino acid sequences of seroin1, seroin2 and seroin3 are shown in SEQ ID NO.1, SEQ ID NO.2 and SEQ ID NO.3 respectively; the nucleotide sequences of seroin1, seroin2 and seroin3 are shown in SEQ ID NO.4 , shown in SEQ ID NO.5 and SEQ ID NO.6. Then use the following online software and biological software for sequence analysis:
[0026] Protein signal peptide prediction: SignalP (http: / / www.cbs.dtu.dk / services / SignalP / );
[0027] Protein molecular weight and isoelectric point prediction: ExPASy (http: / / www.expasy.org / tools / );
[0028] Homology comparison software: Clustal X and GeneDoc;
[0029] The analysis results of amino acid sequence length, signal peptide, molecular weight and isoelectric point a...
Embodiment 2
[0034] Embodiment 2, seroin protein truncated synthesis
[0035] Based on previous research results, the three silkworm seroin proteins were divided into N-terminal and C-terminal parts after removing the signal peptide sequence, and the obtained sequences are shown in Table 2 (Dong et al., 2016). The full-length and truncated peptides of seroin protein were obtained by chemical synthesis, and they were named seroin1-N, seroin1-C, seroin2-N, seroin2-C, seroin3-N, and seroin3-C. The relevant information has been listed in Table 1. illustrate.
[0036] Table 2, the amino acid sequence of the truncated body of silkworm seroin protein
[0037]
Embodiment 3
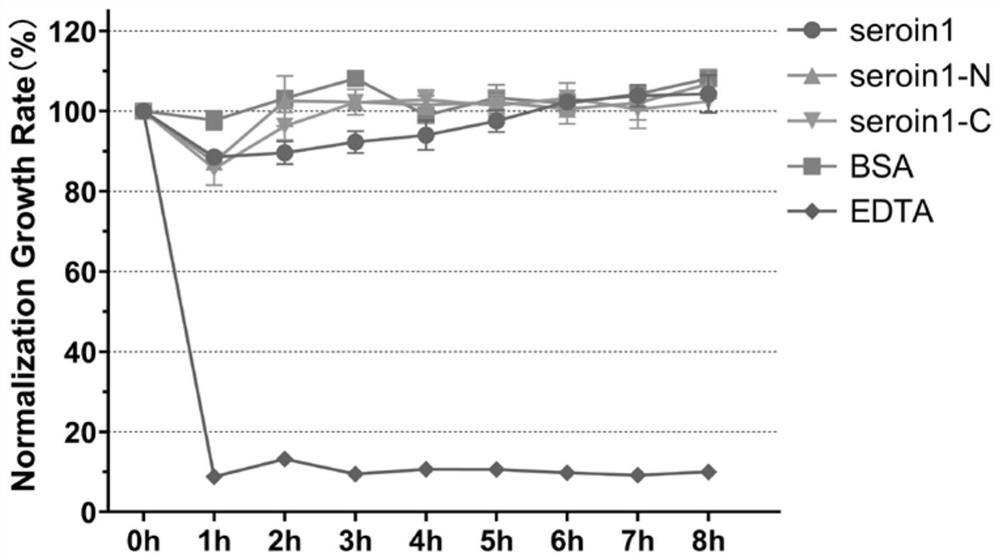
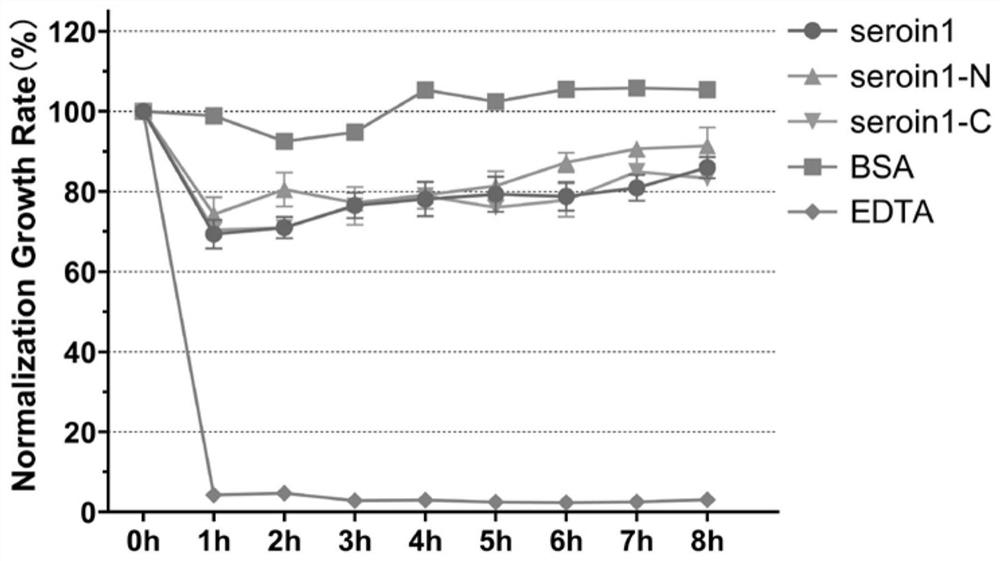
[0038] Example 3, Verification of antibacterial activity of seroin protein truncated peptides
[0039]In order to evaluate the antibacterial activity of different seroin protein peptides, two kinds of bacteria were selected in this experiment: Escherichia coli (Gram-negative bacteria, G-) and Staphylococcus aureus (Staphylococcus aureus, Gram-positive bacteria, G+) were compared with Each seroin protein peptide segment was incubated, and the growth curve of the bacteria was detected by spectrophotometry, and the growth rate of the bacteria relative to the control group was calculated. The specific method is as follows:
[0040] 1) Using sterile phosphate buffer solution (PBS), dissolve a sufficient amount of Seroin protein peptides to a protein concentration of 1 mg / mL;
[0041] 2) Using LB liquid medium, culture the bacteria to OD 600 =0.2-0.3;
[0042] 3) Take 50 μL of protein solution and 150 μL of bacterial solution, add them to each well of a 96-well plate, mix thoroug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com