Preparation method of medical sustained-release gel with microporous structure and cold compress gel for treating dermatitis and eczema
A slow-release gel, microporous structure technology, applied in the field of biomedicine, can solve the problems of long-term use, easy recurrence, short efficacy, etc., and achieve the effects of high frequency of use, difficult recurrence of efficacy, and large toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]A method for preparing an antipruritic, anti-inflammatory and antibacterial cold compress gel is provided. The specific steps are as follows:
[0033](1) Weigh 0.5 g of polyvinyl pyrrolidone and 0.5 g of polyvinyl alcohol into 50 mL of ultrapure water, heat and stir at 50° C. to completely dissolve, forming a 2% polyvinyl pyrrolidone / polyvinyl alcohol solution.
[0034](2) Weigh 2g of chitosan with high degree of deacetylation, dissolve it in 0.1wt% glacial acetic acid, and stir overnight at room temperature to completely dissolve all the chitosan to form a chitosan solution with a mass percentage of 2%.
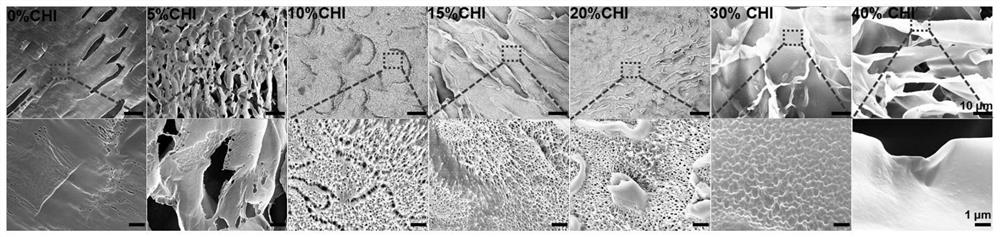
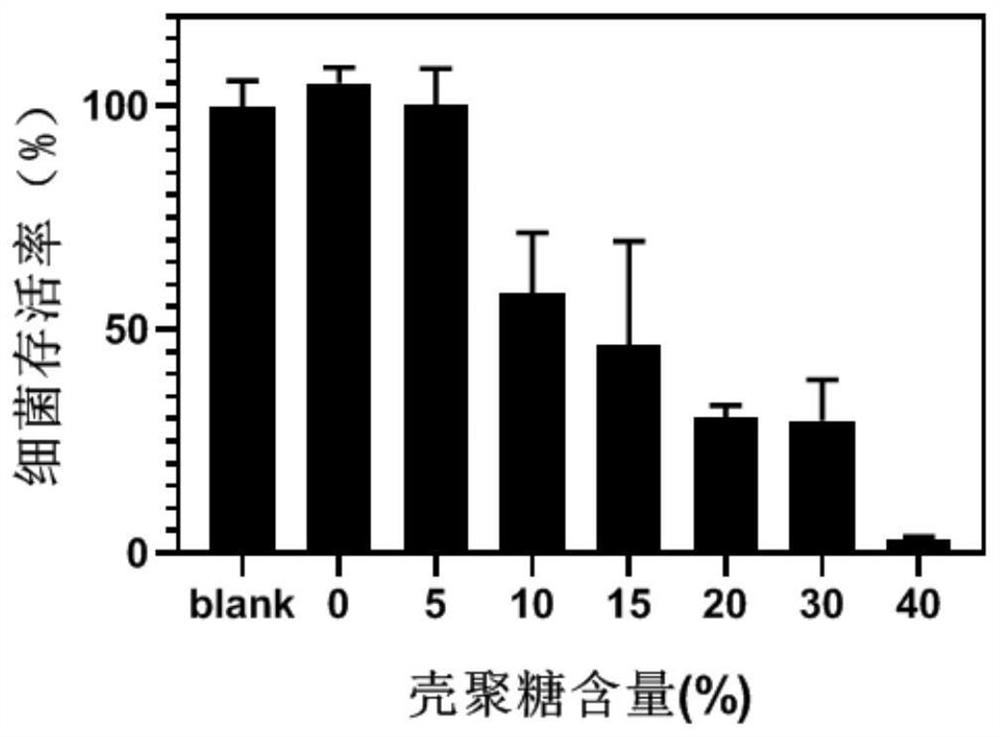
[0035](3) Take an appropriate amount of the above-dissolved polyvinylpyrrolidone / polyvinyl alcohol solution, and add an appropriate volume of the chitosan solution dissolved in the above-mentioned method to obtain the mass fraction of polyvinylpyrrolidone / polyethylene and chitosan. The ratios are 100:0, 95:5, 90:10, 85:15, 80:20, 70:30, 60:40 mixed solutions.
[0036](4) Place each of t...
Embodiment 2
[0039]A method for preparing a medical sustained-release gel with a microporous structure is provided. The specific steps are as follows:
[0040](1) Add 0.5 g of polyvinylpyrrolidone into 50 mL of ultrapure water, stir at room temperature until completely dissolved, and form a polyvinylpyrrolidone solution with a mass percentage of 1%.
[0041](2) Weigh 0.2 g of carboxymethyl cellulose sodium (CMC-Na) and dissolve in ultrapure water, add and stir to completely dissolve it at room temperature, to form a 0.2% carboxymethyl cellulose sodium solution.
[0042](3) Take an appropriate amount of the above-dissolved polyvinylpyrrolidone solution, and add an appropriate volume of the sodium carboxymethylcellulose solution dissolved by the above-mentioned method to make the mass ratio of polyvinylpyrrolidone and sodium carboxymethylcellulose 45:55.
[0043](4) Place the above-mentioned mixed solution in a constant temperature oven, heat at 100°C, and dry until the residual solution is 20% of the initial...
Embodiment 3
[0045]A method for preparing a medical sustained-release gel with a microporous structure is provided. The specific steps are as follows:
[0046](1) Add 0.5 g of polyvinylpyrrolidone into 50 mL of ultrapure water, stir at room temperature until completely dissolved, and form a polyvinylpyrrolidone solution with a mass percentage of 1%.
[0047](2) Weigh 0.2g of sodium carboxymethyl cellulose and dissolve it in ultrapure water, add and stir to completely dissolve it at room temperature, form a 0.2% sodium carboxymethyl cellulose solution, and dissolve it. The sodium carboxymethylcellulose solution is added to the polyvinylpyrrolidone solution in a certain volume ratio, and the mixture is evenly mixed. The mass ratio of the polyvinylpyrrolidone to the sodium carboxymethylcellulose is 1:1.
[0048](3) Weigh 0.2g of chitosan with high degree of deacetylation and dissolve it in 0.1wt% glacial acetic acid, stir overnight at room temperature to completely dissolve all the chitosan and form a chito...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com