Preparation method of aromatic diether dianhydride
A diether dianhydride and aromatic technology, which is applied in the field of preparation of aromatic diether dianhydride, can solve the problems of difficult purification and increased operation complexity, and achieve the effects of simple process, low production cost and short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 bisphenol A type diether dianhydride
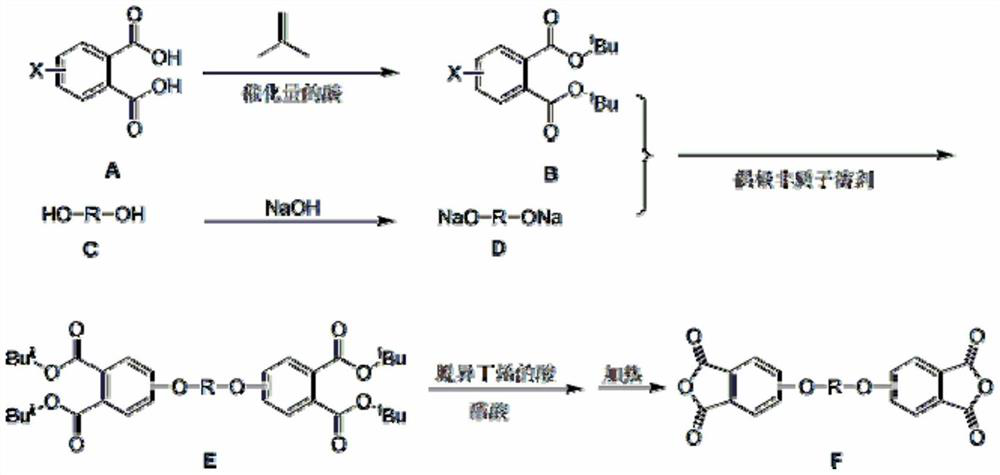
[0030] (1) Mix 4-nitrophthalic acid (46.45g, 0.22mol) with xylene (160mL), concentrated sulfuric acid (1.0mL), heat to 50°C, add dropwise tert-butanol (63.1mL, 0.66mol ), monitored by HPLC until the reaction was complete, cooled to 20-30°C after the reaction was complete, added solid sodium hydroxide (2.0g), and stirred for 30 minutes, evaporated xylene (about 140mL) under reduced pressure, added dimethyl ethyl Amide (200mL), standby;
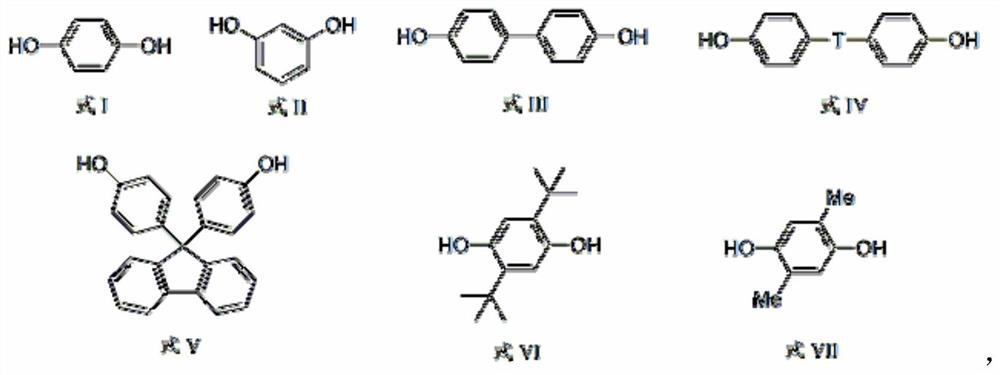
[0031] (2) Mix bisphenol A (22.89g, 0.1mol), sodium hydroxide (8.0g, 0.2mol) and xylene (150mL), reflux under nitrogen atmosphere to remove water to dryness, then distill off xylene (approx. 130mL), cooled to below 100°C to obtain bisphenol disodium salt;
[0032] (3) Add the solution prepared in step (1) to the bisphenol disodium salt prepared in step (2), heat at 140°C for 5 hours, evaporate the solvent (about 200mL) under reduced pressure after the reaction, and ...
Embodiment 2
[0035] The preparation of embodiment 2 bisphenol A type diether dianhydrides
[0036] (1) Mix 4-chlorophthalic acid (44.13g, 0.22mol) with xylene (160mL), concentrated sulfuric acid (1.0mL), heat to 60°C, feed isobutylene gas, and monitor with HPLC until the reaction is complete , cooled to 20-30°C, added solid sodium hydroxide (2.0g), and stirred for 30 minutes, evaporated xylene (about 140mL) under reduced pressure, added dimethylformamide (200mL), set aside;
[0037] (2) Mix bisphenol A (22.89g, 0.1mol), sodium hydroxide (8.0g, 0.2mol) and xylene (150mL), reflux under nitrogen atmosphere to remove water to dryness, then distill off xylene (approx. 130mL), cooled to below 100°C to obtain bisphenol disodium salt;
[0038] (3) Add the solution prepared in step (1) to the bisphenol disodium salt prepared in step (2), heat at 170°C for 5 hours, evaporate the solvent (about 200mL) under reduced pressure after the reaction, and cool down to 60°C Next, add acetic acid (120mL) and...
Embodiment 3
[0041] Embodiment 3 The preparation of 4,4'-triphenylene diether dianhydride
[0042] (1) Mix 4-chlorophthalic acid (44.13g, 0.22mol) with xylene (160mL), p-toluenesulfonic acid (2.0g), heat to 80°C, feed isobutylene gas, and monitor with HPLC until After the reaction is complete, cool to 20-30°C, add solid sodium hydroxide (2.0 g), and stir for 30 minutes, evaporate xylene (about 140 mL) under reduced pressure, add dimethylacetamide (200 mL), and set aside;
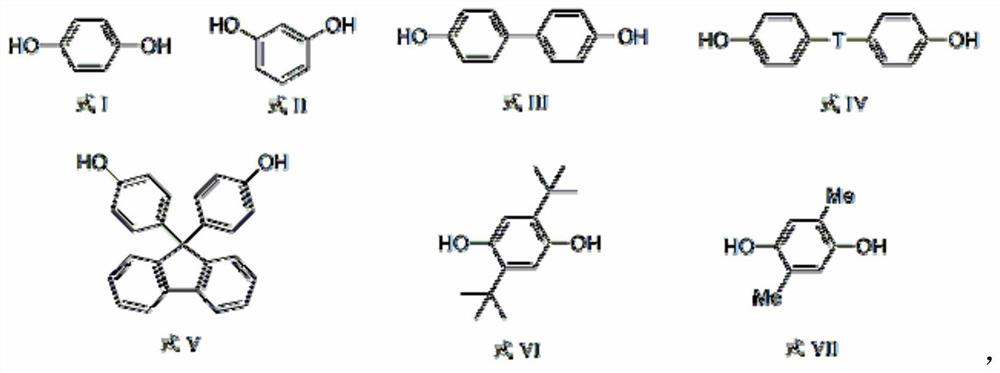
[0043] (2) Hydroquinone (11.0g, 0.1mol), sodium hydroxide (8.0g, 0.2mol) and xylene (150mL) were mixed, refluxed to remove water to dryness under a nitrogen atmosphere, and then evaporated xylene ( about 130mL), lower the temperature to below 100°C to obtain bisphenol disodium salt;
[0044] (3) Add the solution prepared in step (1) to the bisphenol disodium salt prepared in step (2), heat at 150°C for 6 hours, evaporate the solvent (about 200mL) under reduced pressure after the reaction, and cool to 60°C Next, add aceti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com