Visible light regulated dithienylethylene fluorescent molecular switch, its preparation and application
A technology of dithienylethylene and fluorescent molecules, which is applied in the fields of fluorescence/phosphorescence, material analysis through optical means, luminescent materials, etc., can solve the problem of low fluorescent switch ratio and achieve high fluorescent switch ratio and high fluorescence quenching rate , the effect of increasing the contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
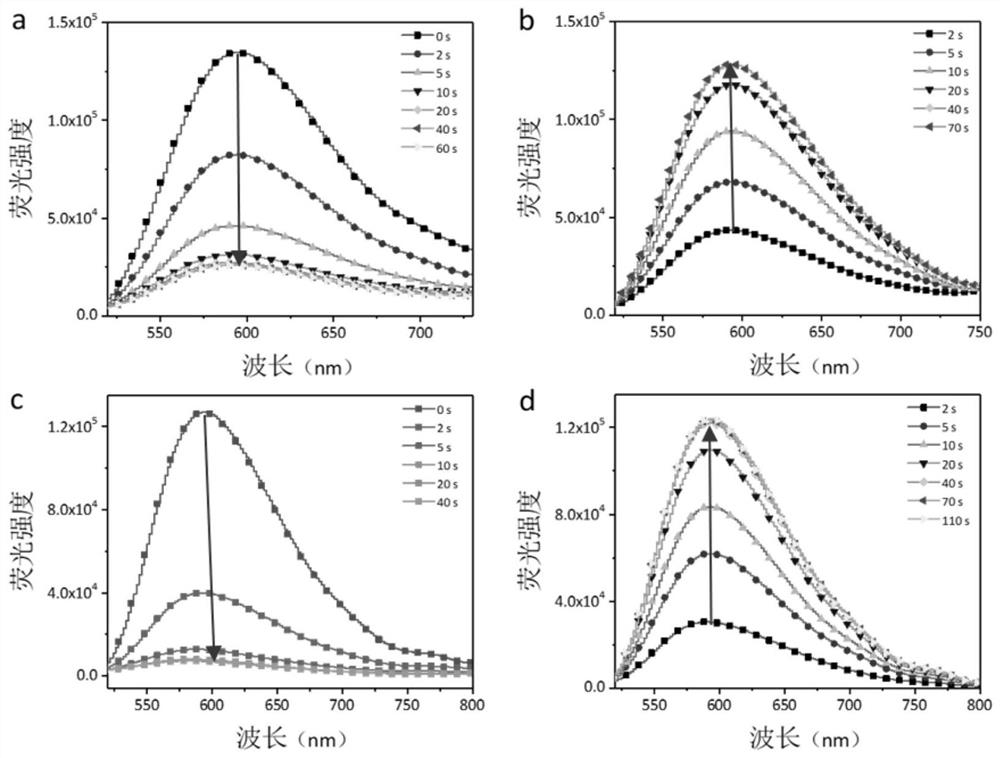
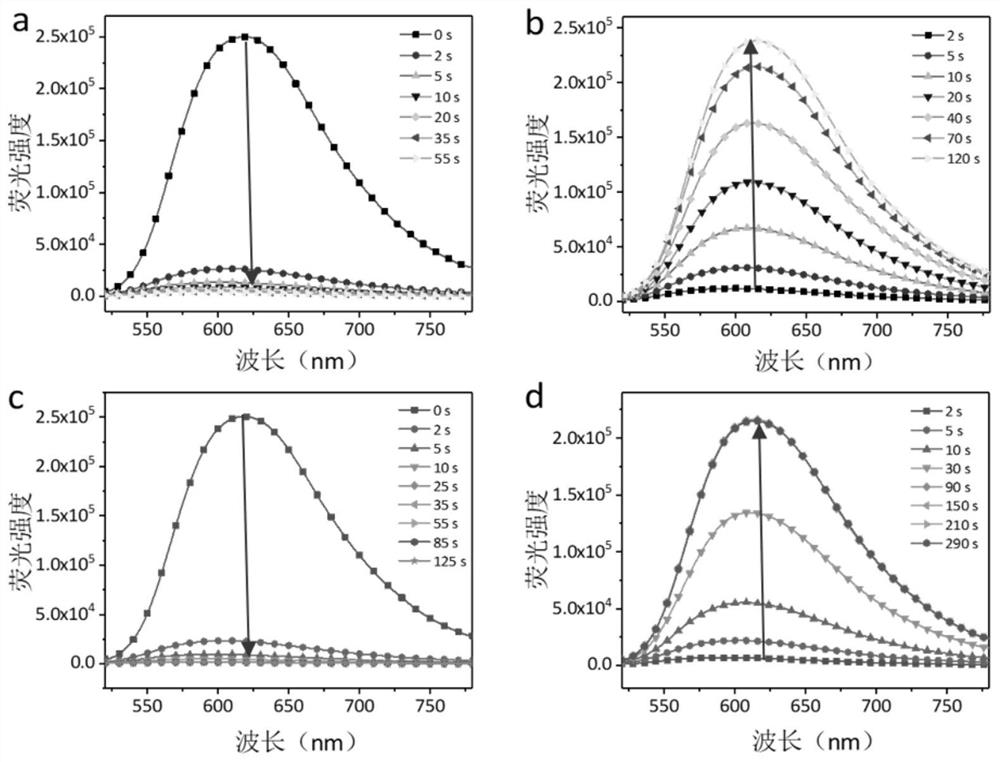
Image
Examples
preparation example Construction
[0068] The invention provides the preparation method of the said dithienylethylene derivative, comprising the following preparation steps:
[0069](1) First, use 3,5-dibromo-2-methylthiapan, n-butyllithium and trimethylchlorosilane as raw materials to obtain 3-bromo-2-methyl-5-trimethyl through substitution reaction base silylthiophene; then use 3-bromo-2-methyl-5-trimethylsilylthiophene, n-butyllithium and perfluorocyclopentene as raw materials to prepare 1,2-bis(2-methyl base-5-trimethylsilylthiophen-3-yl)perfluorocyclopentene, and finally with 1,2-bis(2-methyl-5-trimethylsilylthiophen-3-yl)perfluorocyclopentene Pentene, anhydrous tetrahydrofuran and NBS were used as raw materials, and reacted at room temperature for 16 hours in the dark to obtain a compound, 1,2-bis(5-bromo-2-methylthiophen-3-yl) perfluorocyclopentene, namely Br -DTE-Br.
[0070] (2) With Br-DTE-Br, with R 1 and R 2 The aniline compound and triphenylphosphine of the group are the main reaction raw mater...
Embodiment 1
[0087] A kind of diarylethene fluorescent molecular switch shown in formula (three), wherein R 1 and R 2 for Its name is abbreviated as TPA-AC-DTE-Ph-O-PMI (or DTE-PMI-1), and its synthetic route is as follows figure 1 shown, including the following steps:
[0088] (1) References for the synthesis process of Br-DTE-Br (Li Chong, Synthesis, properties and applications of diarylethene fluorescent molecular switches [D]. Wuhan, Huazhong University of Science and Technology Wuhan Optoelectronics National Research Center, 2015: 28- 32).
[0089] (2) Under the protection of nitrogen, Br-DTE-Br (1.5g, 4.75mmol), triphenylphosphine (0.015g, 0.095mmol), 4-ethynyltriphenylamine (0.77g, 4.75mmol), redistilled 25mL of triethylamine and 120mL of redistilled tetrahydrofuran were quickly poured into a 250mL two-necked flask, and Pd(PPh 3 ) 2 Cl 2 (0.01g, 0.238mmol) and copper iodide (0.108g, 0.095mmol), carefully evacuate nitrogen several times to remove oxygen, and react at 90°C for 2...
Embodiment 2
[0098] A kind of diarylethene fluorescent molecular switch shown in formula (four), its name is abbreviated as TPA-AC-DTE-PMI (or DTE-PMI-2), wherein R 1 and R 2 for Its synthetic route is as follows Figure 6 shown, including the following steps:
[0099] (1) The synthesis of Br-DTE-Br is shown in Example 1;
[0100] (2) The synthesis process of TPA-AC-DTE-Br is shown in Example 1;
[0101] (3) PMI-Br (0.80g, 1.42mmol), bis-boronic acid pinacol ester (0.54g, 2.2mmol) and potassium acetate (0.36g, 3.6mmol), divalent palladium catalyst were added to a 50mL two-necked flask PdCl 2 (dppf) (50mg, 0.072mmol) and redistilled 1,4-dioxane (50mL), stirred and mixed evenly, evacuated nitrogen three times to remove oxygen, heated to 90°C for 16h. Quench the reaction solution, after cooling to room temperature, use CH 2 Cl 2 Extraction, the organic phases were combined and washed three times with distilled water, washed with anhydrous MgSO 4 After drying, suction filtration, spi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com