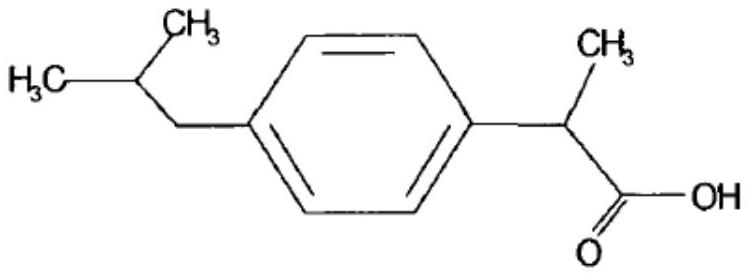
Ibuprofen sustained-release capsule and preparation method thereof
A slow-release capsule and capsule technology, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of difficult and complete drug release, irregular drug release, and increased cost. Achieve the effect of being beneficial to drug protection, high production efficiency, and avoiding high temperature operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Prescription
[0032]
[0033] 2) Preparation process
[0034] Mix the ethanol solution of ibuprofen and the slow-release carrier ethanol solution of the prescribed amount, the solid content of the ethanol solution is 8%, spray dry, set the inlet air temperature at 95°C, the atomization pressure at 1.4bar, and the spray speed at 8r / min. The outlet air temperature is 52°C. The prepared solid dispersion is added with zinc stearate, mixed evenly, and filled into capsules.
Embodiment 2
[0036] 1) Prescription
[0037]
[0038] 2) Preparation process
[0039] Mix the ethanol solution of ibuprofen and the sustained-release carrier ethanol solution of the prescribed amount, the solid content of the ethanol solution is 8%, spray dry, set the inlet air temperature at 100°C, the atomization pressure at 1.5bar, and the spray speed at 8r / min, The outlet air temperature is 58°C. Add micropowder silica gel to the prepared solid dispersion, mix well, and fill capsules.
Embodiment 3
[0041] 1) Prescription
[0042]
[0043] 2) Preparation process
[0044] Mix the ibuprofen ethanol solution and slow-release carrier ethanol solution of prescription quantity, the solid content of ibuprofen ethanol solution is 8%, the solid content of slow-release carrier ethanol solution is 10%, spray dry, set air inlet temperature 100 ℃, atomization pressure 1.5bar, spray speed 9r / min, air outlet temperature 55℃. Add sodium stearate fumarate to the prepared solid dispersion, mix well, and fill capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com