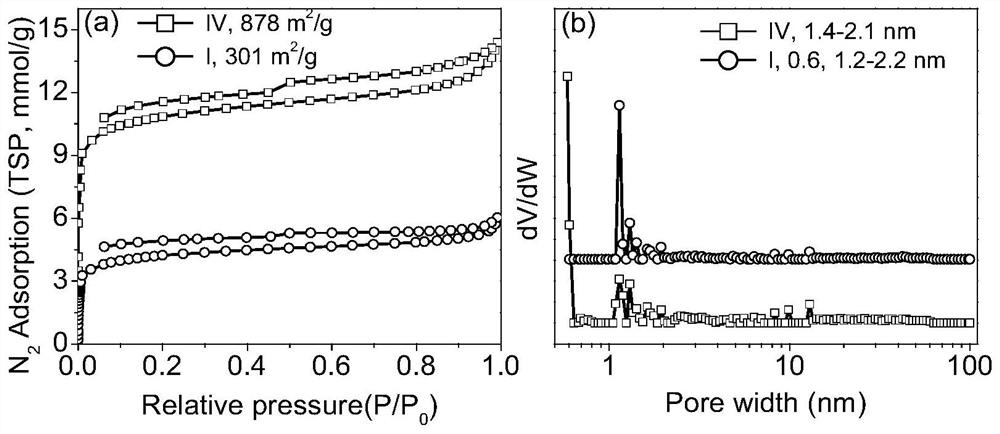
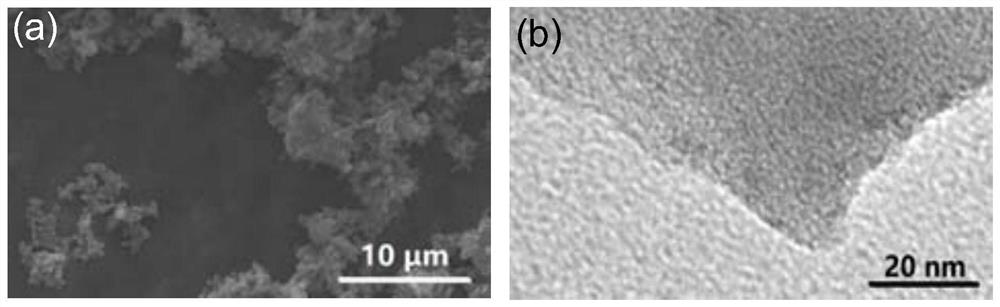
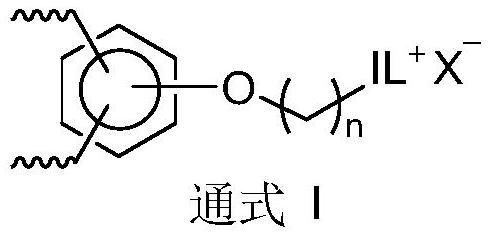
Porous ionic polymer heterogeneous catalyst and method for catalytically synthesizing N-formamide by using porous ionic polymer heterogeneous catalyst
An ionic polymer, heterogeneous catalyst technology, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Repeated use and other problems, to achieve the effects of good physical and chemical stability, low cost of raw materials, and simple product separation and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: preparation of porous ionic polymer catalyst
[0038] (1) Add 5mmol of 1,1'-bi-2-naphthol, 20mmol of anhydrous ferric chloride and 20mL of anhydrous 1,2-dichloroethane into a 50mL Schlenk tube, and repeatedly Replace the air in the tube, add 20mmol of dimethylformal under nitrogen condition, after sealing, put the reaction system at room temperature and stir for 30min, then heat the oil bath to 100°C to carry out the first contact reaction, after 24h, cool and pass Filtration, washing, Soxhlet extraction and vacuum drying to obtain a porous organic polymer containing phenolic hydroxyl groups;
[0039] (2) Add 300 mg of phenolic hydroxyl-containing porous organic polymer obtained in step (1), 414 mg of potassium carbonate and 634 mg of bromobutyltriethylammonium bromide to 15 mL of anhydrous N,N'-dimethylformamide , heated to 140°C and continued to stir and react for 24 hours, filtered the obtained precipitate, washed the filter cake with demethanol, aceto...
Embodiment 2
[0040] Embodiment 2: preparation of porous ionic polymer catalyst
[0041] (1) Add 10mmol of 2,3-dihydroxynaphthalene, 20mmol of anhydrous ferric chloride and 40mL of anhydrous chloroform into a 100mL Schlenk tube, and repeatedly replace the air in the tube with nitrogen. Add 20mmol of dimethylformal, after sealing, place the reaction system at room temperature and stir for 30min, then heat the oil bath to 80°C for the first contact reaction, after 36h, cool, filter, wash, Soxhlet extraction and vacuum Dry to obtain a porous organic polymer containing phenolic hydroxyl groups;
[0042] (2) Add 150 mg of the porous organic polymer containing phenolic hydroxyl groups obtained in step (1), 360 mg of sodium carbonate and 380 mg of 1-(4-bromoethyl) pyridinium chloride into 20 mL of anhydrous tetrahydrofuran, and heat to 100 Continue to stir and react at ℃ for 48 hours, filter the obtained precipitate, wash the filter cake with demethanol, acetone and ionized water successively for...
Embodiment 3
[0043] Embodiment 3: preparation of porous ionomer catalyst
[0044] (1) Add 2mmol of 4,4′-isopropylidene biphenol, 10mmol of anhydrous aluminum trichloride and 20mL of anhydrous dichloromethane into a 100mL Schlenk tube, and repeatedly replace the air in the tube with nitrogen, After sealing under nitrogen, the reaction system was stirred at room temperature for 30 minutes, and then heated to 80 ° C in an oil bath for the first contact reaction. After 48 hours, it was cooled, filtered, washed, Soxhlet extracted and dried in vacuo to obtain Porous organic polymers of phenolic hydroxyl groups;
[0045] (2) Add 200 mg of the phenolic hydroxyl-containing porous organic polymer obtained in step (1), 580 mg of potassium carbonate and 620 mg of bromobutyltriethylammonium bromide to 25 mL of anhydrous N,N'-dimethylformamide , heated to 80°C and continued to stir and react for 48 hours, filtered the obtained precipitate, washed the filter cake with demethanol, acetone and ionized wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com