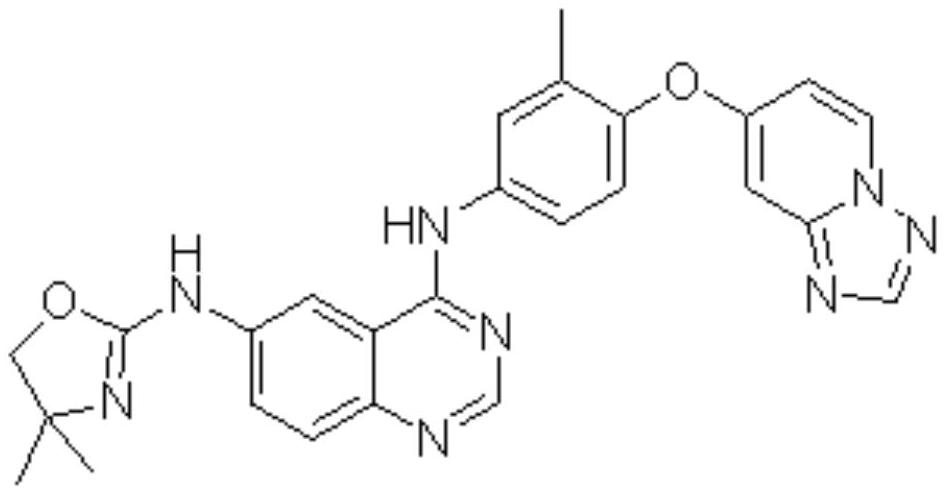
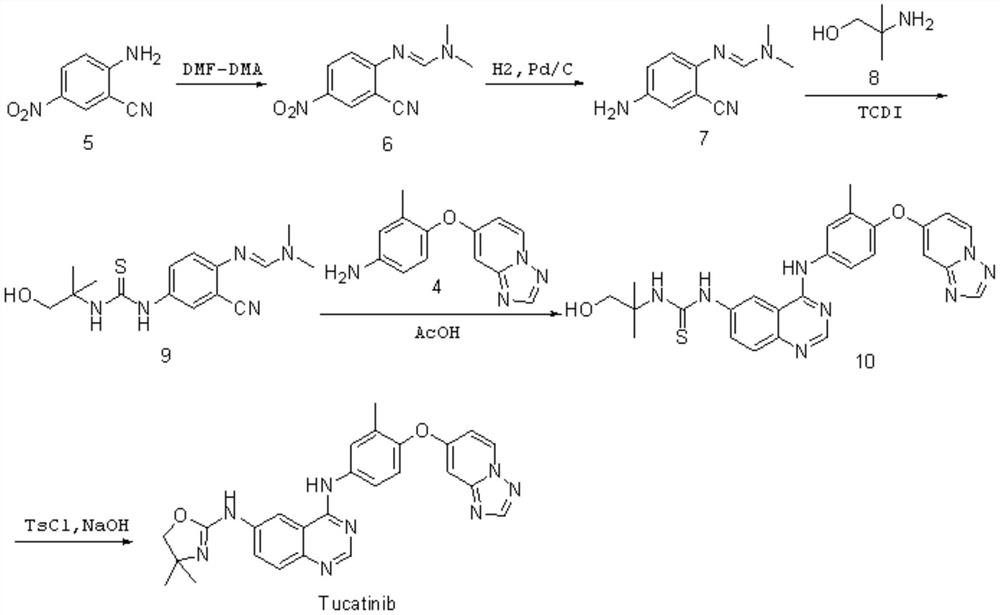
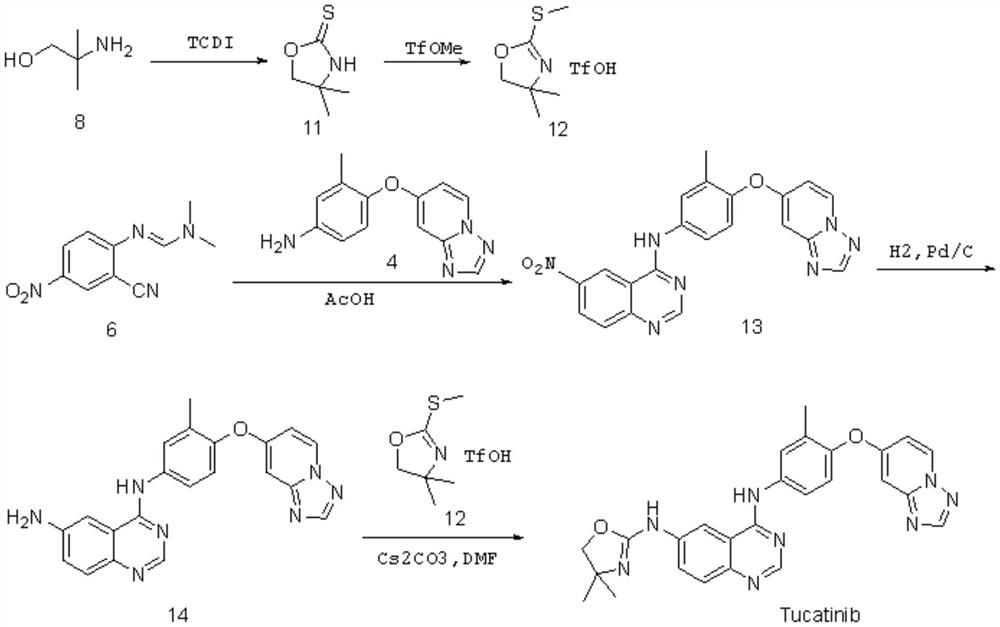
Preparation method of tucatinib
A technology of tucatinib and hydroxyl, which is applied in the field of preparation of tucatinib, can solve problems such as product purity and yield impact, failure to meet environmental protection requirements, abnormal odor, etc., achieve simple and environmentally friendly routes, reduce operation difficulty and Environmental requirements, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under nitrogen protection, dissolve 0.1mol of 4-hydroxy-6-chloroquinazoline into 200ml of N,N-dimethylformamide, add 0.003mol of cuprous bromide, 0.11mol of potassium phosphate, and 0.006mol of N-methylnicotinamide , 0.15 mol of 2-amino-4,4-dimethyl-4,5-dihydrooxazole, and then the reaction solution was heated to 120° C. for 20 hours. Cool, concentrate under reduced pressure to remove the solvent, add 200ml of dichloromethane and saturated aqueous ammonium chloride solution, separate the layers, wash the organic phase with saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure, and recrystallize the residue with methanol and water to obtain a white solid product with a yield of 82.1%.
[0030] 4-Hydroxy-6-(4,4-dimethyl-4,5-dihydrooxazol-2-amino)quinazoline 0.05mol and Carter condensing agent BOP0.06mol were dissolved in 200ml of acetonitrile, added N, N-dimethylaminopyridine 0.075mol, stirred at room temperature for 0.5 hours, then a...
Embodiment 2
[0032]Under nitrogen protection, 0.1 mol of 4-hydroxy-6-chloroquinazoline was dissolved in 200 ml of ethylene glycol monomethyl ether, and 0.001 mol of cuprous bromide, 0.14 mol of potassium phosphate, 0.004 mol of N-methylbenzamide, 0.18 mol of 2-amino-4,4-dimethyl-4,5-dihydrooxazole, and then the reaction solution was heated to 100° C. for 25 hours. Cool, concentrate under reduced pressure to remove the solvent, add 200ml of dichloromethane and saturated aqueous ammonium chloride solution, separate the layers, wash the organic phase with saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure, and recrystallize the residue with methanol and water to obtain a white solid product with a yield of 86.2%.
[0033] 4-Hydroxy-6-(4,4-dimethyl-4,5-dihydrooxazol-2-amino)quinazoline 0.05mol and Carter condensing agent BOP0.075mol were dissolved in 200ml tetrahydrofuran, and diiso Propylethylamine 0.06mol, stirred at 40°C for 0.7 hours, then added 4-(...
Embodiment 3
[0035] Under nitrogen protection, 0.1 mol of 4-hydroxy-6-chloroquinazoline was dissolved in 200 ml of a mixed solvent of dimethyl sulfoxide and ethylene glycol, and 0.005 mol of cuprous bromide, 0.1 mol of potassium phosphate, and N-methyl Nicotinamide 0.002mol, 2-amino-4,4-dimethyl-4,5-dihydrooxazole 0.1mol, and then the reaction solution was heated to 150°C for 10 hours. Cool, concentrate under reduced pressure to remove the solvent, add 200ml of dichloromethane and saturated aqueous ammonium chloride solution, separate the layers, wash the organic phase with saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure, and recrystallize the residue with methanol and water to obtain a white solid product with a yield of 84.7%.
[0036] Dissolve 0.05mol of 4-hydroxy-6-(4,4-dimethyl-4,5-dihydrooxazol-2-amino)quinazoline and 0.1mol of Carter’s condensing agent BOP into 200ml of ethylene glycol dimethyl ether Add 0.1 mol of triethylamine, stir the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com