6''-O-succinyl daidzin derived from biological method and application of 6''-O-succinyl daidzin derived from biological method to preparation of neuroprotective drugs and health products
A technology of neuroprotection and succinyl, applied in the field of medicine, can solve problems such as limited application, low production efficiency, and difficulty in commercial promotion and application, and achieve significant drug effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Fermentation of Bacillus amyloliquefaciens FJ18 and preparation of resting cells
[0045] Fermentation of Bacillus amyloliquefaciens FJ18 was inoculated into the seed medium: yeast extract 5.0g / L, peptone 10.0g / L, NaCl 10.0g / L, pH7.0, cultured at 30°C, 200rpm for 12 hours. Expansion medium, fermentation medium, its components and contents are: sucrose 20g / L, yeast powder 15g / L.KH 2 PO 4 1.0g / L, CaCl 2 0.8g / L. The pH was adjusted to 8.0 with NaOH. The seed solution was inoculated into the expansion medium and the fermentation medium at 0.5% (v / v), and cultivated at 30° C. and 200 rpm for 12 hours. After centrifuging at 10000rpm for 15 minutes, the bacterial cells were collected, washed 1-2 times with physiological saline, and the resting cells of Bacillus amyloliquefaciens FJ18 were obtained.
Embodiment 2
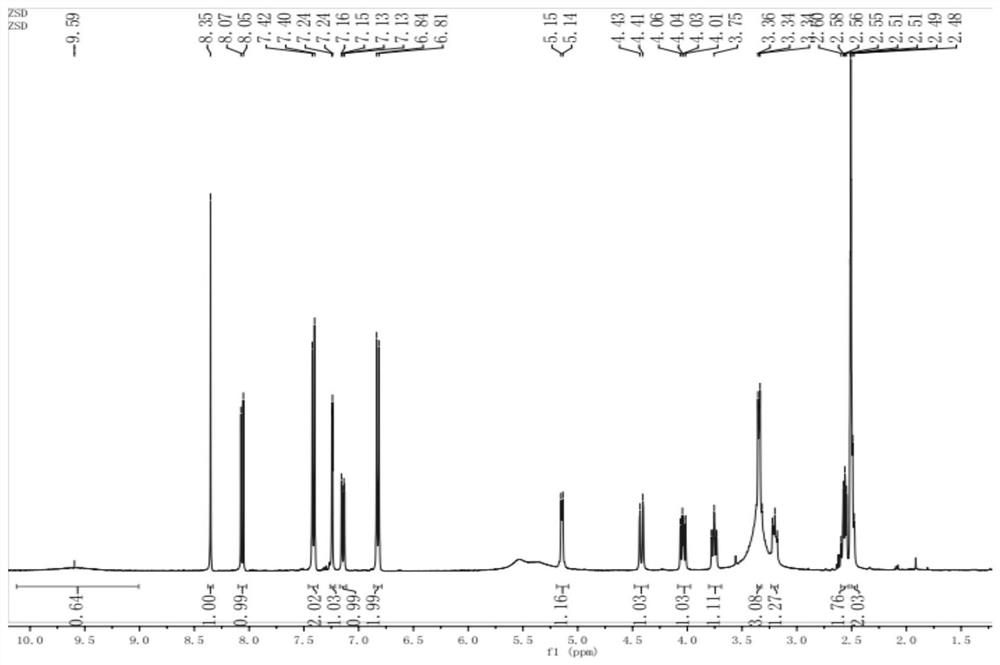
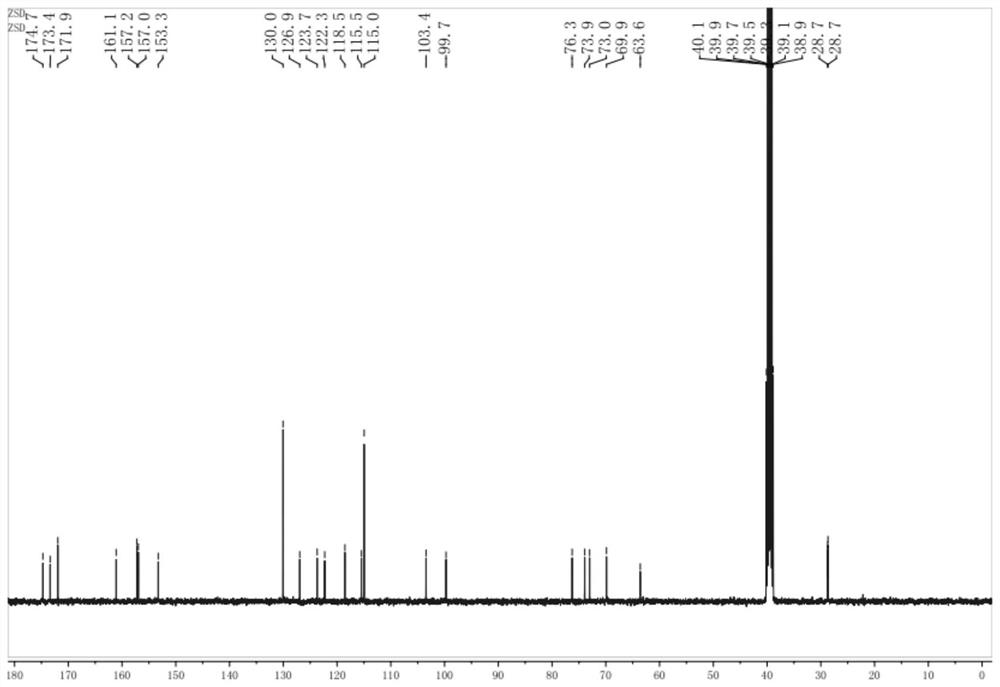
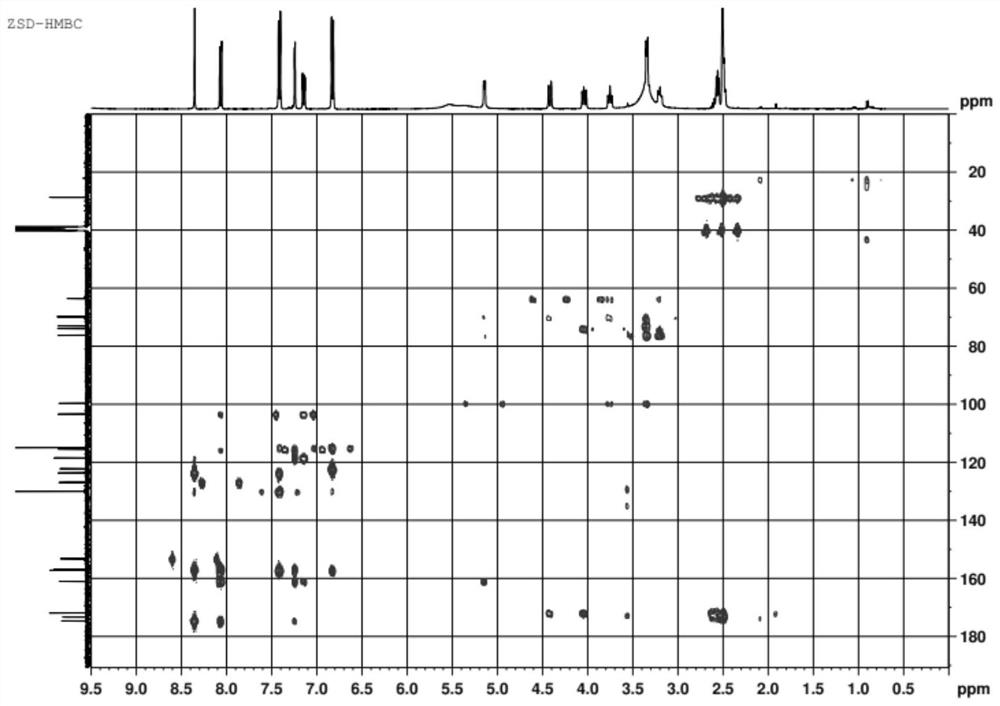
[0047] The thalline cell fermentation broth in Example 1 was filtered to obtain wet thalline. The raw material solution, that is, the reaction solution, is prepared with dimethyl sulfoxide, daidzein, sucrose, and phosphate buffer. The ratio of organic solvent dimethyl sulfoxide in the reaction solution is 20% (v / v), daidzein 0.5g / L, the molar concentration of phosphate buffer is 150mmol / L, the pH of phosphate buffer is 8.0, and the sucrose concentration is 50g / L L. Disperse the above-obtained wet bacteria in the reaction solution, add it to the reactor, cultivate it at 30°C and 200rpm for 12 hours, centrifuge at 10000rpm for 10 minutes to obtain the supernatant of the reaction solution, and measure the conversion rate of daidzein by HPLC detection and analysis was 96.2%.
[0048] The product is separated with a macroporous resin, and an appropriate amount of resin is taken, soaked in ethanol for 24 hours, and then resin fragments and sundries are removed. Wet Packing Rins...
Embodiment 3
[0055] The thalline cell fermentation broth in Example 1 was filtered to obtain wet thalline. The raw material solution, that is, the reaction solution, is prepared with dimethyl sulfoxide, daidzein, sucrose, and phosphate buffer. The ratio of organic solvent dimethyl sulfoxide in the reaction solution is 15% (v / v), daidzein 1.0g / L, the molar concentration of phosphate buffer is 125mmol / L, the pH of phosphate buffer is 8.0, and the sucrose concentration is 25g / L L. Disperse the above-obtained wet bacteria in the reaction solution, add it to the reactor, cultivate it at 30°C and 200rpm for 12 hours, centrifuge at 10000rpm for 10 minutes to obtain the supernatant of the reaction solution, and measure the conversion rate of daidzein by HPLC detection and analysis was 95.6%.
[0056] The product is separated with a macroporous resin, and the method is the same as in Example 2; the product detection method is the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com