Panaxadiol compound as well as preparation method and medical application thereof
A technology of panaxadiol and pharmaceutical preparations, applied in the field of biomedicine, can solve problems such as incomplete cure, achieve good neuroprotective effect, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) 6 mmol p-nitrophenyl chloroformate in dichloromethane solution (6 mmol / 10 mL) was slowly added to 1 mmol panaxadiol in dichloromethane solution (1 mmol / 1 mL) under ice bath , then 2 mmol of N,N-lutidine (DMAP) and 3 mmol of triethylamine were added, and the reaction was carried out overnight at room temperature;
[0024] 2) After the completion of the reaction, purify by silica gel chromatography to obtain Intermediate A with a yield of 93%;
[0025] 3) Take 1 mmol of Intermediate A and 5 mmol of methylpiperazine into 3 mL of ethanol, and react at room temperature for 8 h;
[0026] 4) After the reaction is completed, ethyl acetate is added for extraction, washed with saturated brine, suction filtered, dried, and subjected to column chromatography to obtain compound Q4.
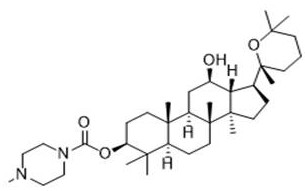
[0027] The resulting compounds are named:
[0028] (3 S ,5 R ,8 R ,9 R ,10 R ,12 R ,13 R ,14 R ,17 S )-12-hydroxy-4,4,8,10,14-pentamethyl-17-(( R )-2,6,6-trimethyltetrahydro-2 H -pyran-2...
Embodiment 2
[0035] Pharmaceutical composition: 1000 tablets each containing 100 mg of active ingredient Formulation: 100 g of the present compound Q4, 2 g of hydroxypropyl methylcellulose, 10 g of wheat starch, 100 g of sucrose, 6 g of magnesium stearate ; The dose to be used should be adapted to the nature and severity of the disease, the route of administration and the age and weight of the patient. The daily dose varies from 0.1 mg to 1.0 g and can be administered in one or several doses.
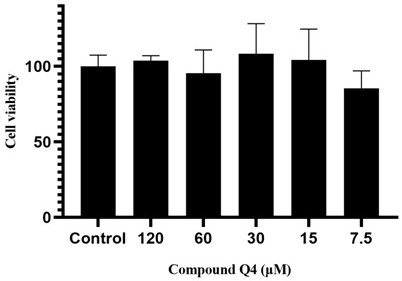
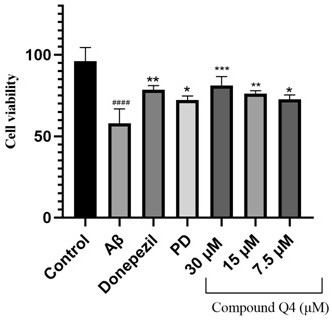
[0036] The medical use of the compounds of the present invention is further demonstrated by the following tests:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com