Method for establishing fingerprint spectrum of volatile components of musk wind-dispelling and pain-relieving paste, standard fingerprint spectrum and application thereof
A technology of volatile components and fingerprints, applied in the field of identification of traditional Chinese medicine preparations, to achieve the effects of good solution stability and durability, quality assurance, and improved quality control level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
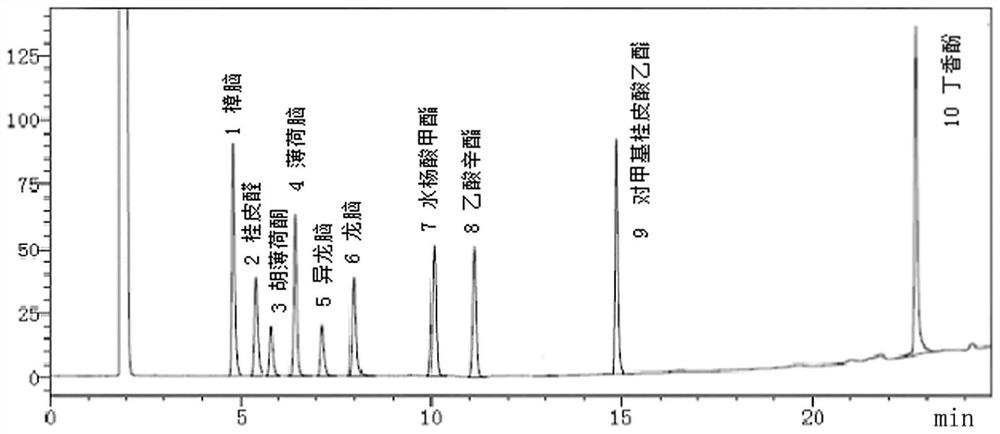
[0040] Embodiment 1: the establishment of standard fingerprint
[0041] Taking the composition of Musk Zhuifeng Zhipain Ointment (rubber plaster, approval number: Z20027408, specification: 7cm*10cm, amount of ointment (drug content) per tablet: not less than 1.12g) composition as an example, the effect on fingerprint The establishment method of the spectrum and the standard fingerprint spectrum will be explained. In this plan, the samples of Musk Zhuifeng Zhipain Ointment include the rubber plaster of Musk Zhuifeng Zhifeng Pain Cream, the gel plaster of Musk Zhuifeng Zhifeng Pain Ointment or the powdery premixed plaster of Musk Zhuifeng Zhifeng Pain Ointment. In this embodiment, the sample of Musk Zhuifeng Zhitong Ointment is a rubber plaster of Musk Zhuifeng Zhitong Ointment.
[0042] 1. Instruments and reagents
[0043] (1) Shimadzu Gas Chromatograph (GC-2014)
[0044] (2) Shimadzu electronic balance (ATY224)
[0045] (3) Musk Zhuifeng Pain Relief Ointment (self-produced...
Embodiment 2
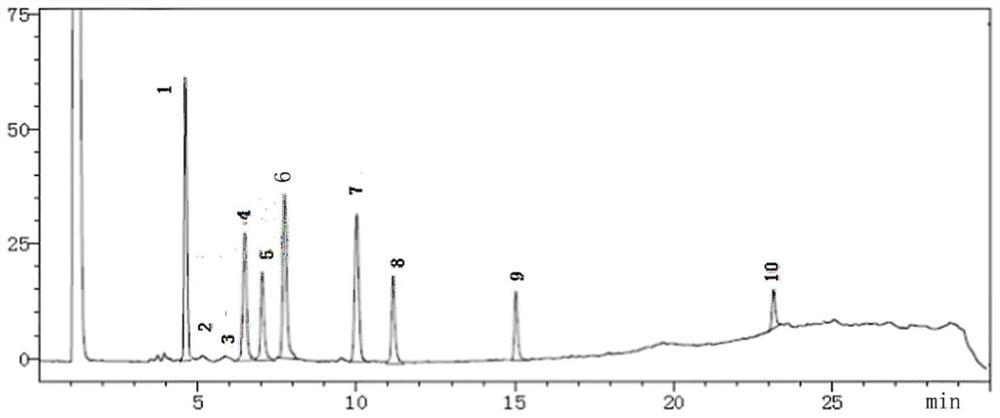
[0055] Embodiment 2: Utilize the standard fingerprint spectrum of establishment to carry out product quality appraisal
[0056] The test sample of this embodiment is Musk Zhuifeng Pain Relief Ointment (rubber plaster, approval number: Z20027408, specification: 7cm*10cm, content of ointment: not less than 1.12g), and its preparation method is according to the publication number: Chinese patent of CN200610079120.0.
[0057] (1) Detection method: chromatographic conditions: Shimadzu gas chromatograph (GC-2014), chromatographic column: polyethylene glycol nitrobenzene is a capillary column (HP-FFAP, column length is 30m, inner diameter is 0.32mm, film thickness is 0.25μm); carrier gas: N 2 ;Column temperature: the initial temperature is 130°C, keep it for 20min, raise the temperature to 195°C at a rate of 7.5°C per minute, keep it for 20min; inlet temperature: 250°C; detector temperature: 250°C.
[0058] (2) Preparation of reference substance solution: get camphor reference subs...
Embodiment 3
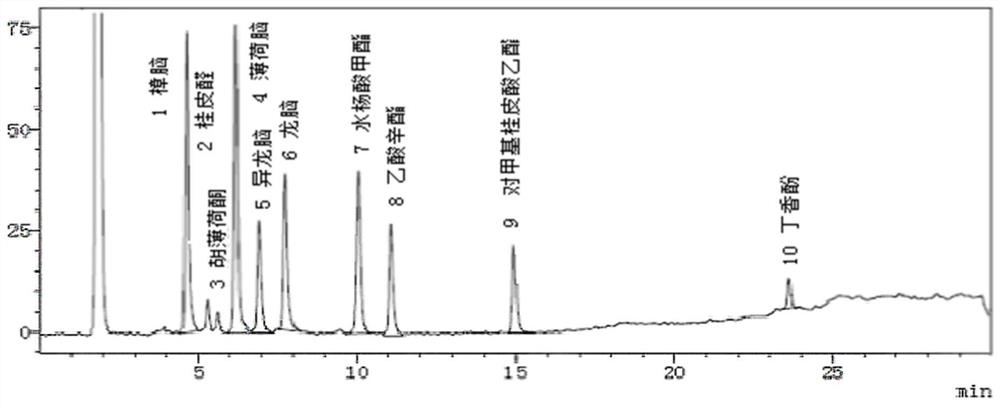
[0062] Embodiment 3: Utilize the standard fingerprint spectrum of establishment to carry out product quality appraisal
[0063] The test sample in this example is Musk Zhuifeng Zhitong Ointment (powder premixed plaster), which is prepared according to the Chinese patent publication number: CN201810344858.8.
[0064] (1) Detection method: chromatographic conditions: Shimadzu gas chromatograph (GC-2014), chromatographic column: polyethylene glycol nitrobenzene is a capillary column (HP-FFAP, column length is 30m, inner diameter is 0.32mm, Film thickness is 0.25μm); Carrier gas: N2; Column temperature: initial temperature is 130°C, keep for 20min, increase temperature to 195°C at a rate of 7.5°C per minute, keep for 20min; inlet temperature: 250°C; detector temperature : 250°C.
[0065] (2) Preparation of reference substance solution: get camphor reference substance, isoborneol reference substance, borneol reference substance, menthol reference substance, pulegone reference subs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com