4-position iodoindole compound and preparation method thereof
An indole compound and compound technology, applied in organic chemistry, drug combination, bone disease, etc., can solve problems such as difficult large-scale application, fire, explosion, etc., and achieve the effects of less side reactions, high product purity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
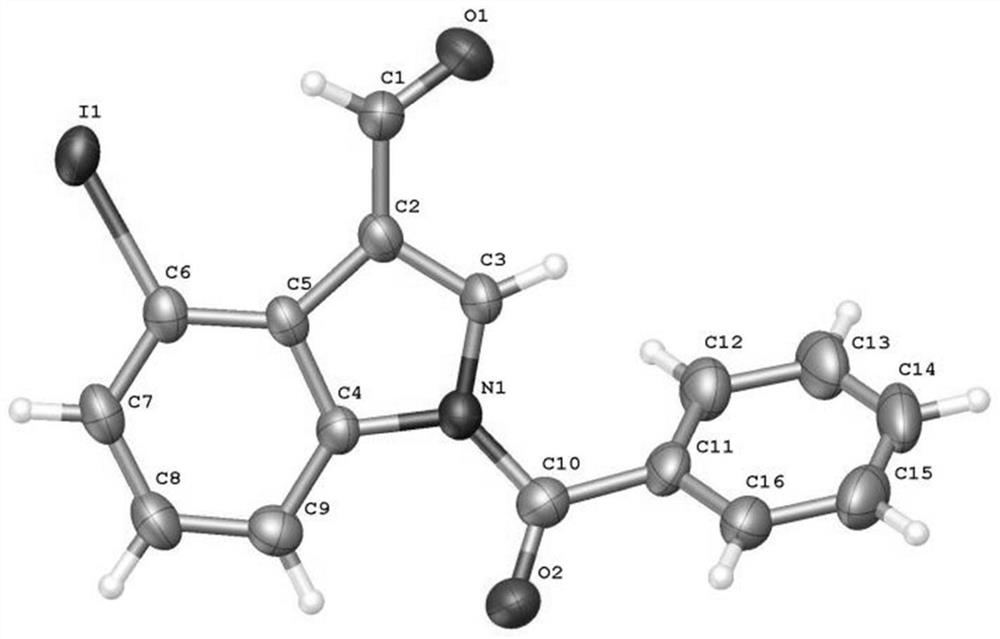
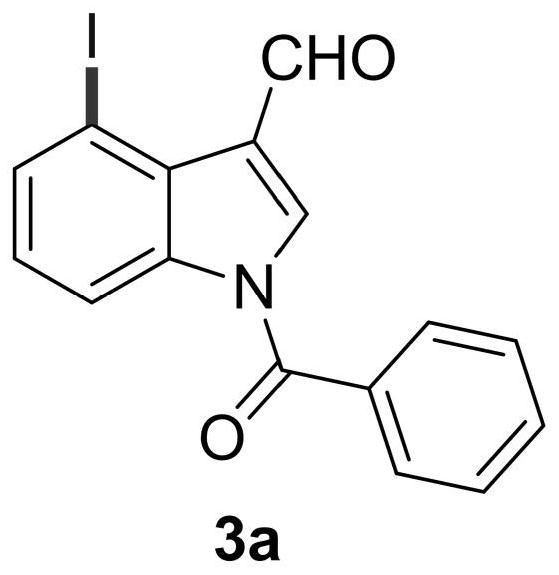
[0049] Add N-benzoylindole-3-carbaldehyde (0.2mmol), N-iodosuccinimide (0.24mmol), catalyst palladium acetate (10mol%), temporary directing group 2 respectively in the reaction test tube successively. -Amino-4-trifluoromethylbenzoic acid (45 mol%), acid additive trifluoroacetic acid (10.0 equiv), and finally the solvent 1,2-dichloroethane (1 mL), and the reaction tube was sealed with a rubber stopper. Place the test tube in an oil bath at 70°C and heat it with stirring for about 24 hours. During the reaction, TLC is used to detect that the reaction is complete. During the post-treatment, the solvent was spin-dried first, and the pure product N-benzoyl-4-iodoindole-3-carbaldehyde compound 3a was directly separated by silica gel column chromatography.
[0050]
[0051] Compound 3a, yield: 54%; yellow solid; melting point 173-175°C; 1 H NMR (400MHz, CDCl 3 )δ11.24(s,1H),8.48(d,J=8.4Hz,1H),8.10(s,1H),7.91(d,J=7.6Hz,1H),7.74(dd,J=7.2,1.2 Hz, 2H), 7.69(t, J=7.6Hz, 1H), 7.58(t,...
Embodiment 2
[0054] The reactants are N-acetylindole-3-carbaldehyde and N-chlorosuccinimide, and the product is N-acetyl-4-iodoindole-3-carbaldehyde compound 3b.
[0055]
[0056] N-acetyl-4-iodoindole-3-carbaldehyde compound 3b, yield: 40%; white solid; melting point 186-187°C; 1 HNMR (400MHz, CDCl 3 )δ11.24(s,1H),8.56(d,J=8.4Hz,1H),8.24(s,1H),7.86(d,J=7.6Hz,1H),7.12(t,J=8.0Hz, 1H), 2.72(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ186.0, 168.7, 137.3, 136.4, 131.3, 130.3, 127.1, 122.0, 116.7, 83.1, 23.9; HRMS(pos.ESI): m / z[M+H] + forC 11 h 9 INO 2 calcd: 313.9672, found: 313.9690.
Embodiment 3
[0058] The reactants are N-benzyloxycarbonyl indole-3-carbaldehyde and N-chlorosuccinimide, and the product is N-benzyloxycarbonyl-4-iodoindole-3-carbaldehyde compound 3c.
[0059]
[0060] N-benzyloxycarbonyl-4-iodoindole-3-carbaldehyde compound 3c, yield: 39%; yellow solid; melting point 119-121°C; 1 H NMR (400MHz, CDCl 3 )δ11.20(s,1H),8.41(s,1H),8.34(d,J=8.4Hz,1H),7.83(d,J=7.6Hz,1H),7.49–7.42(m,5H), 7.09(t,J=8.0Hz,1H),5.48(s,2H); 13 C NMR (100MHz, CDCl 3 )δ185.8,149.6,137.0,136.0,134.0,131.9,130.4,129.3,129.0,128.9,126.6,121.5,115.4,83.3,70.1; HRMS(pos.ESI):m / z[M+H] + for C 17 h 13 INO 3 calcd: 405.9935, found: 405.9908.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com