Azine hydrazine compound containing diethylamine as well as preparation method and application thereof
A technology of diethylamine and compounds, which is applied in the field of azinehydrazine compounds and their preparation, can solve problems such as limited application and limited solubility, and achieve the goals of increasing Stokes shift, preventing self-absorption, and strong binding ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] According to the following synthetic route, specifically synthesize the following compounds:
[0077]
[0078] (1) Synthesis of compound 2
[0079] A mixture of compound 1 (2 mmol) and excess hydrazine hydrate was stirred under reflux for 4 hours. After the reaction was completed, the solvent and the remaining hydrazine hydrate were removed by rotary evaporation to obtain compound 2 as a transparent oil with a yield of 100%.
[0080] (2) Synthesis of compound DPNAP
[0081] Compound 2 (1 mmol) and Compound 3 (1.5 mmol) were stirred under reflux for 4 hours. After the reaction was completed, it was separated with a chromatographic silica gel column to obtain a yellow solid compound DPNAP (an azine hydrazine compound containing diethylamine) with a yield of 81.2%.
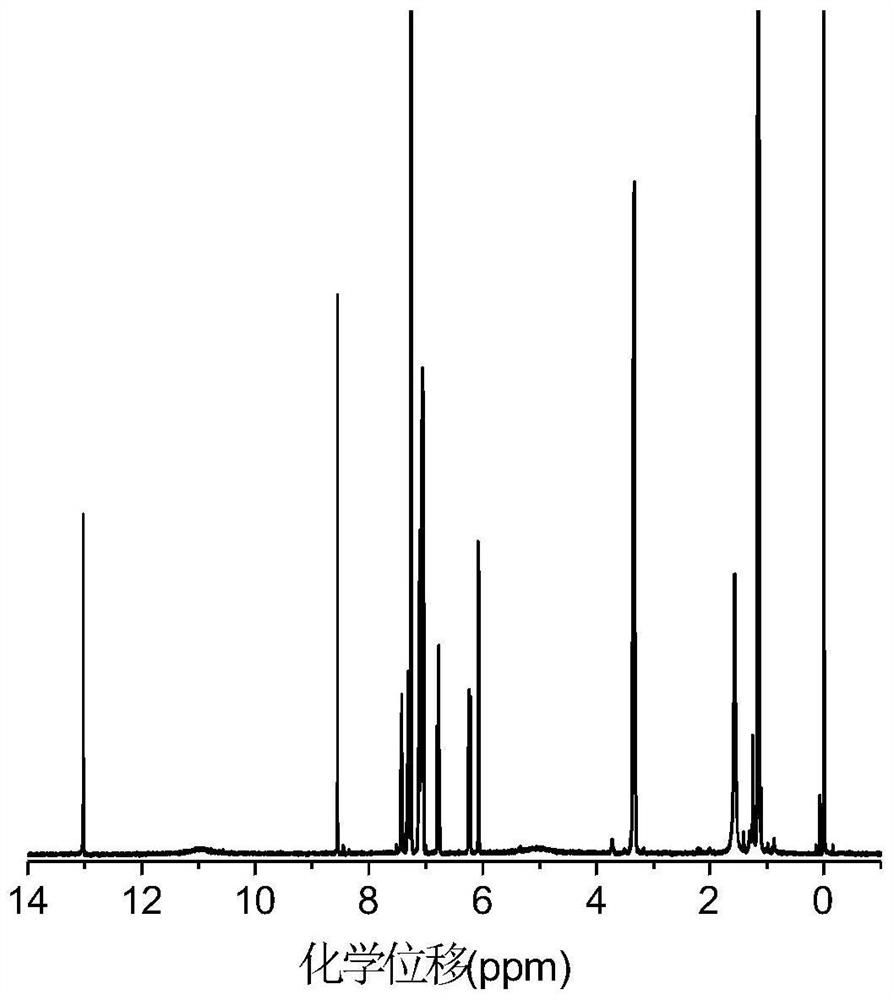
[0082] figure 1 The hydrogen spectrum of DPNAP proves the correctness of its structure.
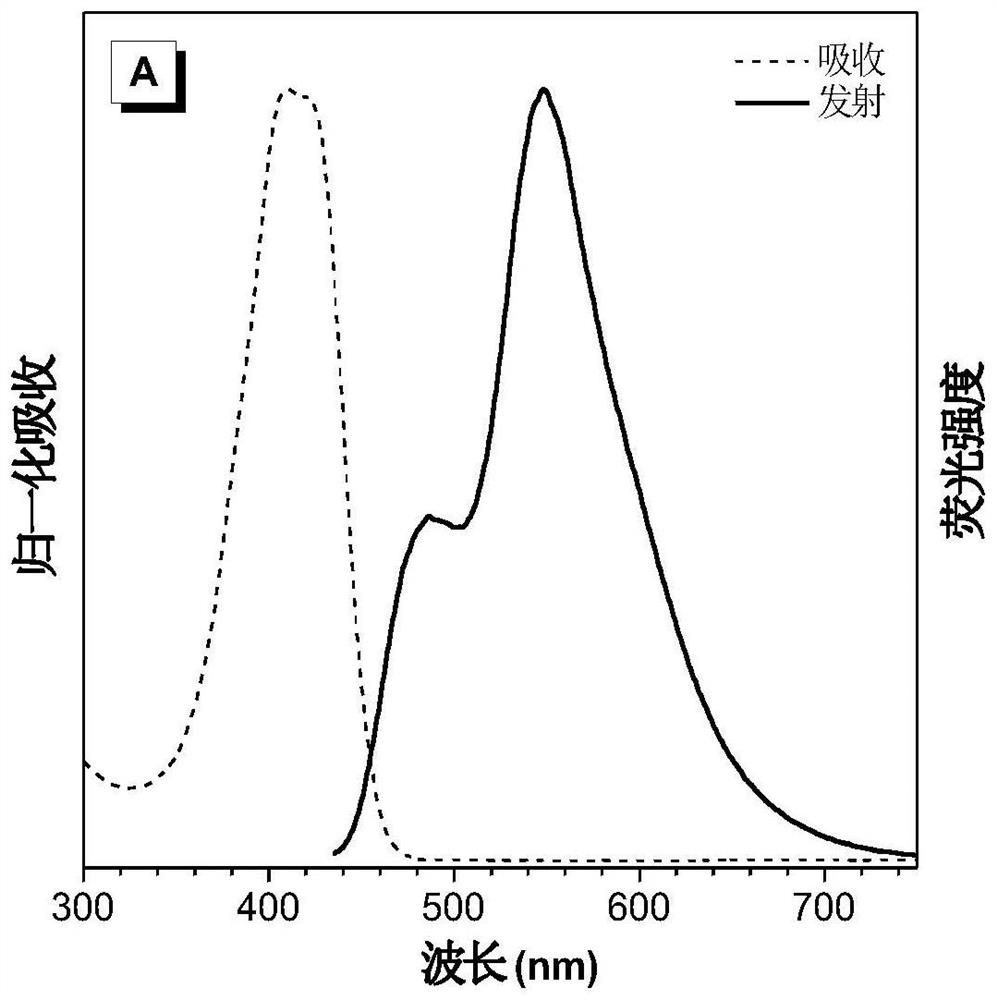
[0083] figure 2 for DPNAP in H 2 Normalized UV absorption spectrum and fluorescence emission spectrum in O / T...
Embodiment 2
[0087] Embodiment 2: compound is used for identifying and killing bacteria in embodiment 1
[0088] (1) Bacteria Imaging Experiment
[0089] a. Bacteria (Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Enterococcus faecalis, Pseudomonas aeruginosa, Candida albicans, Saccharomyces cerevisiae) were inoculated in 5 mL of culture medium, and cultured at 37° C. for 12 hours. Afterwards, the bacterial classification was centrifuged at 7100 rpm for 1 minute, while washing three times with phosphate buffered saline (PBS, 10 mM, pH=7.4), the supernatant was discarded, and the remaining bacterial classification was suspended in PBS, and then diluted at 600 nm to 1.0 optical density (OD 600 = 1.0). For mixed bacteria samples, Candida albicans, Escherichia coli and Staphylococcus aureus were mixed together.
[0090] b. The strains were co-stained with 5 μM DPNAP in PBS buffer solution at 37°C for 20 minutes, and then centrifuged at 7100 rpm for 1 minute. The stained strains...
Embodiment 3
[0110] According to the following synthetic route, specifically synthesize the following compounds:
[0111]
[0112] (1) Synthesis of Compound 5
[0113] A mixture of compound 4 (2 mmol) and excess hydrazine hydrate was stirred at reflux for 4 hours. After the reaction was completed, the solvent and remaining hydrazine hydrate were removed by rotary evaporation to obtain compound 5 with a yield of 100%.
[0114] (2) Synthesis of compound DO-DPAS
[0115] Compound 5 (1 mmol) and compound 3 (1.5 mmol) were stirred under reflux for 4 hours. After the reaction was completed, it was separated with a chromatographic silica gel column to obtain a yellow solid compound DO-DPAS with a yield of 75%.
[0116] Figure 16 It is the hydrogen spectrum of DO-DPAS.
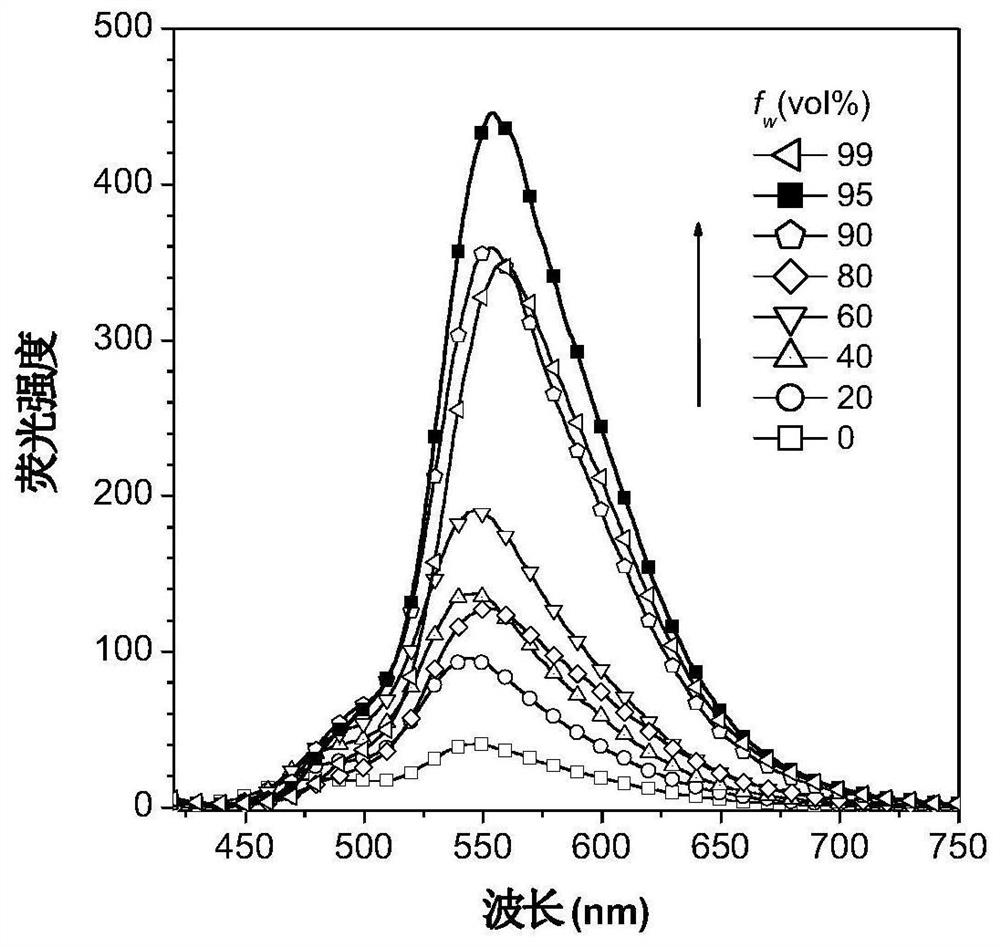
[0117] Figure 17 for DO-DPAS in H 2 Normalized UV absorption and fluorescence emission spectra in O / THF (99:1, v / v) solution. [DO-DPAS] = 10 μM; λ ex = 401 nm. Depend on Figure 17 It can be seen that the maximum e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com