Respiratory tract transmission pathogen enrichment spinning film, sampling mask patch and sampling mask
A technology of spinning membranes and pathogens, which is applied in the field of biomedical materials, can solve problems such as high work risk of throat swab collectors, spread of respiratory infectious diseases, and susceptibility to human factors, so as to improve effectiveness and patient screening efficiency , wide applicability, avoid the effect of throat swab process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
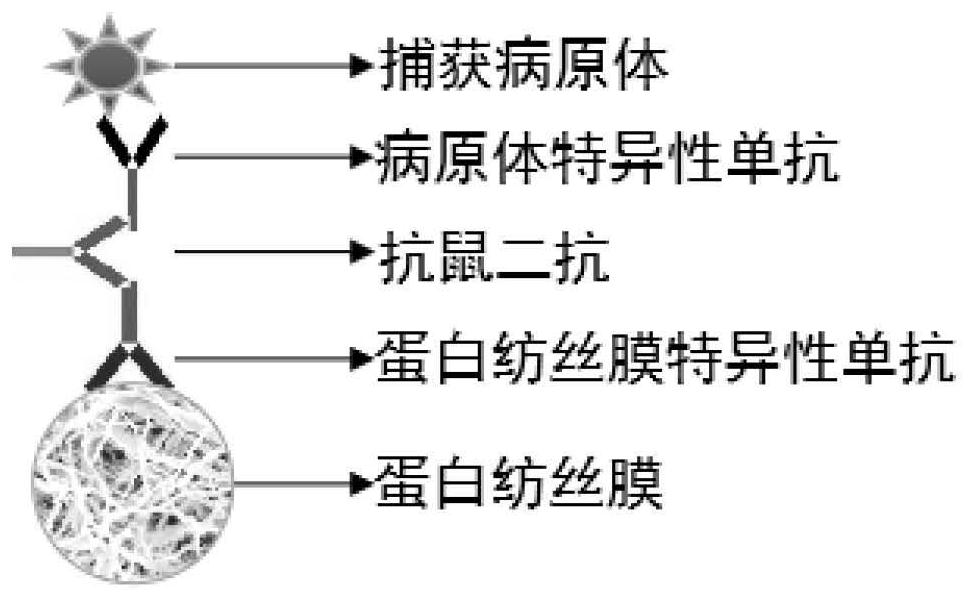
[0033] The preparation of the respiratory tract-transmitted pathogen enrichment spinning membrane includes the following steps: preparing protein spinning membrane mouse monoclonal antibody and pathogen-specific mouse monoclonal antibody, and bridging the two through anti-mouse secondary antibody to obtain a membrane with the ability to capture the respiratory tract The spun membrane with the function of transmitting pathogens: that is, the spun membrane that transmits pathogens in the respiratory tract and enriches them.
[0034] This embodiment also provides the respiratory tract transmission pathogen enrichment sampling mask patch and sampling mask prepared according to the respiratory tract transmission pathogen enrichment spinning membrane. In the solution of the present invention, by simultaneously capturing and enriching the pathogens in the exhaled breath of the wearer of the mask, the complicated throat swab process can be avoided, both doctors and patients can be prot...
Embodiment 1
[0037] The preparation of protein spinning membrane comprises the following steps:
[0038] 1. Protein spinning
[0039] Plant protein silk, animal protein silk and insect silk are frozen and ground into ultra-short silk or powder, adding a small amount of polyethylene terephthalate (PET), fully mixed, spinning and doffing and drafting to form a protein spun mesh with a pore size of 50 μm±10 μm.
[0040] 2. Machining
[0041] Cut the protein spinning net into squares of the same size, and lay them vertically to form a non-woven fabric substrate; spray water mist evenly, place the non-woven fabric substrate in the middle of a hot-pressing plate, and perform vertical heat-pressing to form a thickness of 0.5mm ±0.05mm, protein spinning membrane with a porosity of 2% to 5%.
[0042] 3. Assay of protein spinning membrane
[0043]The pore size, thickness and porosity of the protein spinning membrane were tested, and the specific monoclonal antibody was used for indirect ELISA te...
Embodiment 2
[0045] Preparation of mouse monoclonal antibody and anti-mouse secondary antibody:
[0046] 1. The preparation of mouse monoclonal antibody comprises the following steps:
[0047] 1. Mice Immunization
[0048] Protein spinning membrane antigens and pathogen antigens were prepared respectively, and after emulsification, BLAB / C mice were immunized at multiple points through the abdominal cavity and back subcutaneously, for a total of 4 times with an interval of 10 days. Immunization was boosted through the tail vein of rats 2 days before fusion.
[0049] 2. Cell Fusion
[0050] Take the spleen of the immunized mouse, prepare the spleen cell suspension, and mix the mouse spleen cells with the cultured NS1 myeloma cells. Using 50% PEG 4000 as a fusion agent, the mouse splenocytes and NS1 myeloma cells were fused. Fused cells were suspended in selective medium containing 20% newborn bovine serum and cultured for several days.
[0051] 3. Clonal Screening
[0052] The protei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com