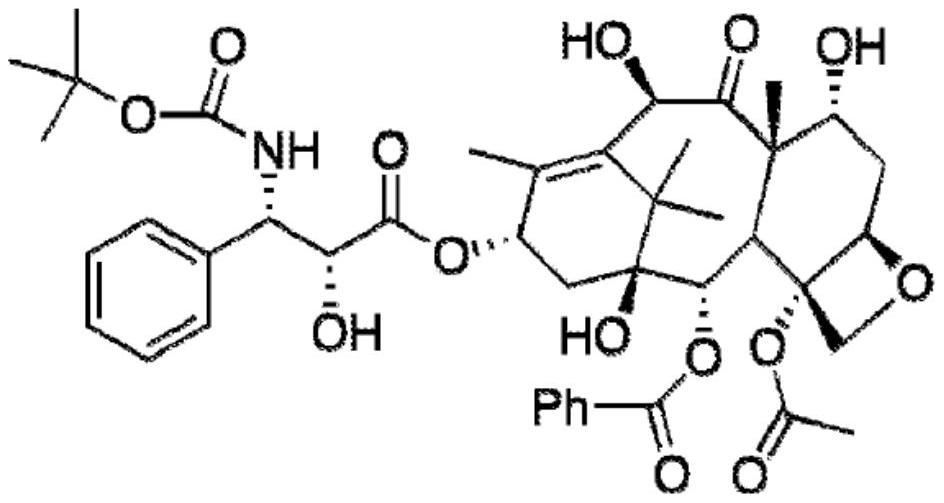
Formulations and compositions of docetaxel
A technology of docetaxel and composition, applied in the field of preparations and compositions containing docetaxel and human serum albumin, can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] Example 1: Compositions comprising docetaxel, human serum albumin (HSA) and amino acids
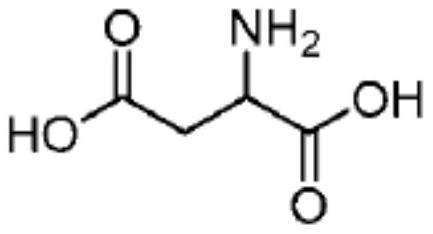
[0242] To 7 different round bottom flasks were added (A) 4.5ml water; (B) 10mg arginine (Arg) dissolved in 4.5ml water; (C) 10mg aspartic acid (Arg) dissolved in 4.5ml water ( Asp); (D) 10 mg cysteine (Cys) dissolved in 4.5 ml water; (E) 10 mg glutamate (Glu) dissolved in 4.5 ml water; (F) 10 mg lysine dissolved in 4.5 ml water Acid (Lys); and (G) 10 mg of Proline (Pro) dissolved in 4.5 ml of water, each of which contained 2.5 ml of 20% human albumin solution (500 mg HSA) for infusion in each round bottom flask. (Note: All amino acids used in the Examples section (all examples) are L-amino acids). After placing the flasks in an ice bath, a mixed solvent (2.5 ml of tert-butanol and 0.5 ml of ethanol) in which docetaxel (5 mg) was dissolved was added dropwise to each of the 7 flasks under stirring, respectively. After the addition was complete, a clear solution was obtained. The...
Embodiment 2
[0248] Example 2: Compositions Comprising Docetaxel, Human Serum Albumin (HSA) and Amino Acids
[0249] To 7 different round bottom flasks were added (A) 4.5ml water; (B) 10mg alanine (Ala) dissolved in 4.5ml water; (C) 10mg asparagine (Asn) dissolved in 4.5ml water ), (D) 10mg cysteine hydrochloride (Cys.HCl) dissolved in 4.5ml water; (E) 10mg glycine (Gly) dissolved in 4.5ml water; (F) 10mg dissolved in 4.5ml water Histidine (His) and (G) 10 mg serine (Ser) dissolved in 4.5 ml of water, each round bottom flask containing 2.5 ml of 20% human albumin solution (500 mg HSA) for infusion. After placing the flasks in an ice bath, a mixed solvent (2.5 ml of tert-butanol and 0.5 ml of ethanol) in which docetaxel (5 mg) was dissolved was added dropwise to each of the 7 flasks under stirring, respectively. After the addition was complete, a clear solution was obtained. The resulting clear aqueous solution was lyophilized overnight to yield a white solid.
[0250] Take 35 mg of ly...
Embodiment 3
[0255] Example 3: Compositions comprising docetaxel, human serum albumin (HSA), amino acids and sugar alcohols
[0256]To 4 different round bottom flasks were added (A) 10 mg aspartic acid (Asp) and 1 mg cysteine hydrochloride (Cys.HCl) dissolved in 4.5 ml water; (B) dissolved in 4.5 ml 10mg aspartic acid (Asp), 1mg cysteine hydrochloride (Cys.HCl) and 25mg mannitol in water; (C) 10mg aspartic acid (Asp), 1mg Cys Cystine hydrochloride (Cys.HCl) and 50 mg mannitol; and (D) 10 mg aspartic acid (Asp), 1 mg cysteine hydrochloride (Cys.HCl) and 100 mg of mannitol, each of which contained 2.5 ml of 20% human albumin solution for infusion (500 mg HSA) in each round bottom flask. After the flask was placed in an ice-water bath at about 10°C, under stirring, a mixed solvent (2.5 ml of tert-butanol and 0.5 ml of ethanol) in which docetaxel (5 mg) was dissolved was added dropwise to each of the four flasks. ). After the addition was complete, a clear solution was obtained. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com