Alpha fetoprotein specific binding polypeptide and application
A technology for specific binding of alpha-fetoprotein, applied in the field of preparation of polypeptides, or functional fragments, or variants thereof, which can solve the problems of complex preparation process, poor thermal stability and high cost of antibody molecules, and achieve low and stable preparation cost Good sex, good effect of peptide-specific affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
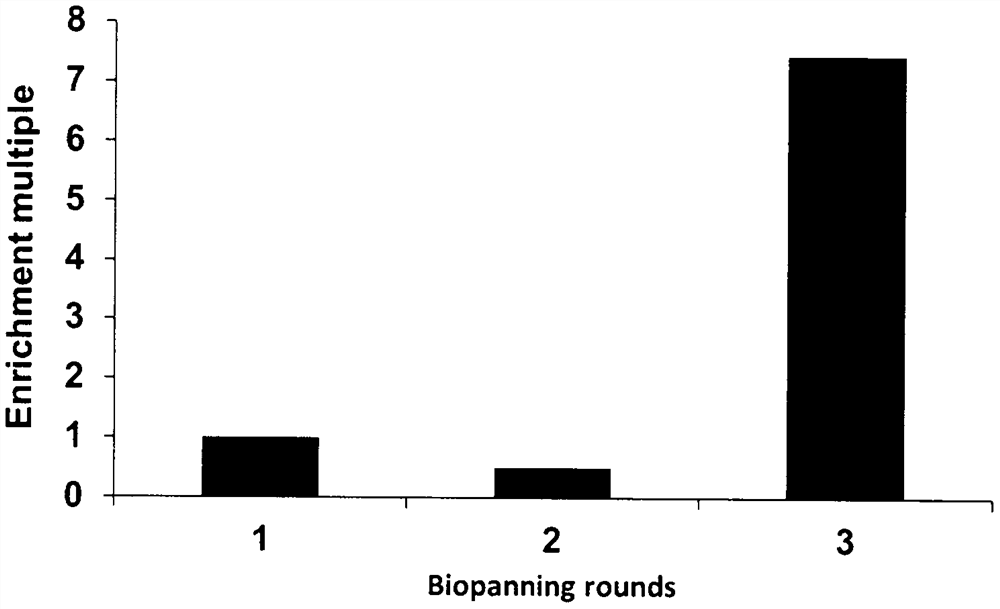
[0031] Panning and identification of alpha-fetoprotein-specific binding polypeptides.
[0032] 1. Mutant construction and single clone selection
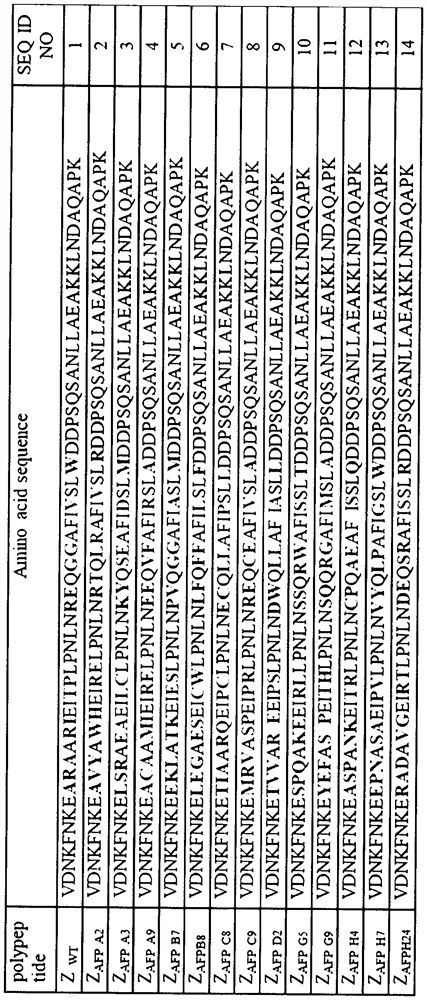
[0033] Aiming at the 9th, 10th, 11th, 13th, 14th, 17th, 18th, 24th, 25th, 27th, 28th, 32nd and 35th amino acid residues of the immunoglobulin binding region polypeptide of staphylococcal protein A, the abbreviation with NNK as the codon is designed and primers, using degenerate primers to obtain the coding gene of the affimer polypeptide library by over-lapPCR. The gene encoding the polypeptide mutant was cloned into a phage display vector, and the recombinant plasmid was electrotransformed into TG1 host cells to obtain a storage capacity of 5×10 7 Affimer polypeptide mutant library. Use the helper phage to display the mutant polypeptides of the affinity body, and then obtain the phage display library of the polypeptide mutants.
[0034] Alpha-fetoprotein extracted from umbilical cord blood (purchased from fitzgerald, Cat. No. 30-1...
Embodiment 2
[0041] Expression and purification of alpha-fetoprotein-specific binding polypeptides.
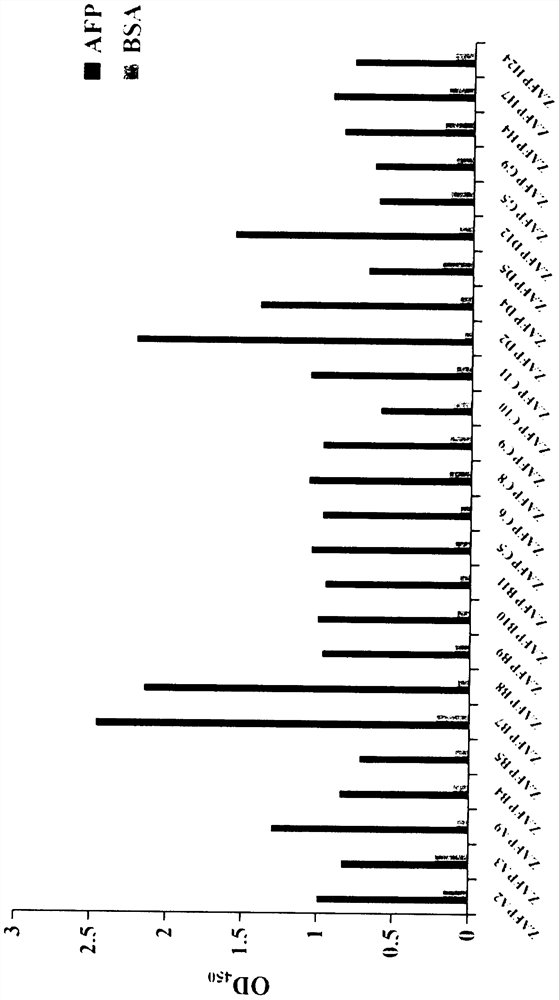
[0042] From the 13 polypeptide mutants specifically binding alpha-fetoprotein identified in Example 1, the following six were selected as objects for further research: ZAFP-D2, ZAFP-A3, ZAFP-B7, ZAFP-G9, ZAFP-A2, ZAFP-B8.
[0043] The expression host is E.coli BL21 (DE3) (preserved in our laboratory), the expression vector is pET-21b (preserved in our laboratory), and the schematic diagram of the recombinant vector is attached. Figure 4 , wherein ZAFP represents the above six different alpha-fetoprotein-specific binding polypeptides, and C represents cysteine.
[0044] Transform the recombinant expression vector with correct sequencing into E.coli BL21(DE3), pick a single clone into 5mL LB / A medium (1g tryptone, 0.5g yeast extract, 1g sodium chloride to 100mL, autoclave ; then add the final concentration of 100 μg / mL ampicillin), 37 ° C, 180 rpm overnight culture; inoculate the culture ...
Embodiment 3
[0047] Analysis of binding properties of alpha-fetoprotein-specific binding polypeptides.
[0048] In this experiment, the six alpha-fetoprotein-specific binding polypeptides expressed and purified in Example 2 were used as further research objects, and their affinity characteristics with alpha-fetoprotein were detected by ELISA method, and CEA, EGF, BSA, and insulin were selected to detect 6 A polypeptide is used as an irrelevant antigen negative control, and the specific operation is as follows:
[0049] Dilute AFP / CEA / EGF / BSA / insulin with pH 9.6 carbonate buffer to 10 μg / mL, coat 96-well ELISA plate at 4°C overnight; block with 3% skimmed milk powder-PBS, 37°C for 2h; add band ZAFP-D2, ZAFP-A3, ZAFP-B7, ZAFP-G9, ZAFP-A2, and ZAFP-B8 six recombinant proteins with HA tags were incubated at 37°C for 2 h; diluted HA antibody (purchased from Sangon Bioengineering Co., Ltd. company, Cat. No. D191044), incubated at 37°C for 2 h; added diluted HRP-labeled rabbit anti-mouse seconda...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com