4-CNAB and preparation method thereof
A 4-CNAB and solution technology, applied in the field of 4-CNAB and its preparation, can solve the problems of large loss, difficult to remove, and low overall yield in the purification process, and achieve the effect of improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
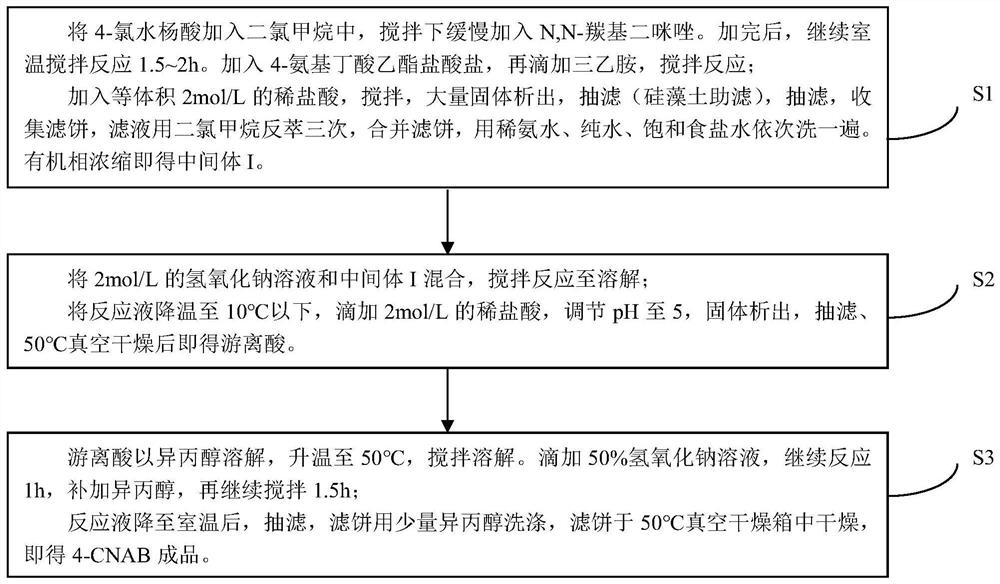
[0039] Such as figure 1 Shown, a kind of preparation method of 4-CNAB comprises the following steps:
[0040] S1: Weigh 5.0062g of 4-chlorosalicylic acid into a 100mL reaction flask, add 20ml of dichloromethane, add 2.5072g of sodium bentonite, slowly add 5.1772g of N,N-carbonyldiimidazole under stirring, a large amount of gas is generated. After the addition, continue to stir at room temperature for 1.5h. Add 5.3507g of ethyl 4-aminobutyrate hydrochloride, then dropwise add 6ml of triethylamine, stir and react at 25°C for 22h to obtain a reaction solution;
[0041] Transfer the reaction solution into a separatory funnel, add an equal volume of 2mol / L dilute hydrochloric acid to the reaction solution, adjust the pH value of the reaction solution to 4.5-5, and shake it sufficiently to have a large amount of solids precipitate out, then filter with suction (diatomaceous earth filter aid) The filter cake was obtained, and the filtrate was collected; then extracted with dilute a...
Embodiment 2
[0051] S1: Weigh 50g of 4-chlorosalicylic acid into a 1L reaction bottle, add 250ml of dichloromethane, add 50g of sodium bentonite, slowly add 50g of N,N-carbonyldiimidazole under stirring, a large amount of gas is generated. After the addition, continue to stir at room temperature for 2h. Add 50 g of ethyl 4-aminobutyrate hydrochloride, then dropwise add 50 ml of triethylamine, stir and react at 20° C. for 30 h to obtain a reaction solution.
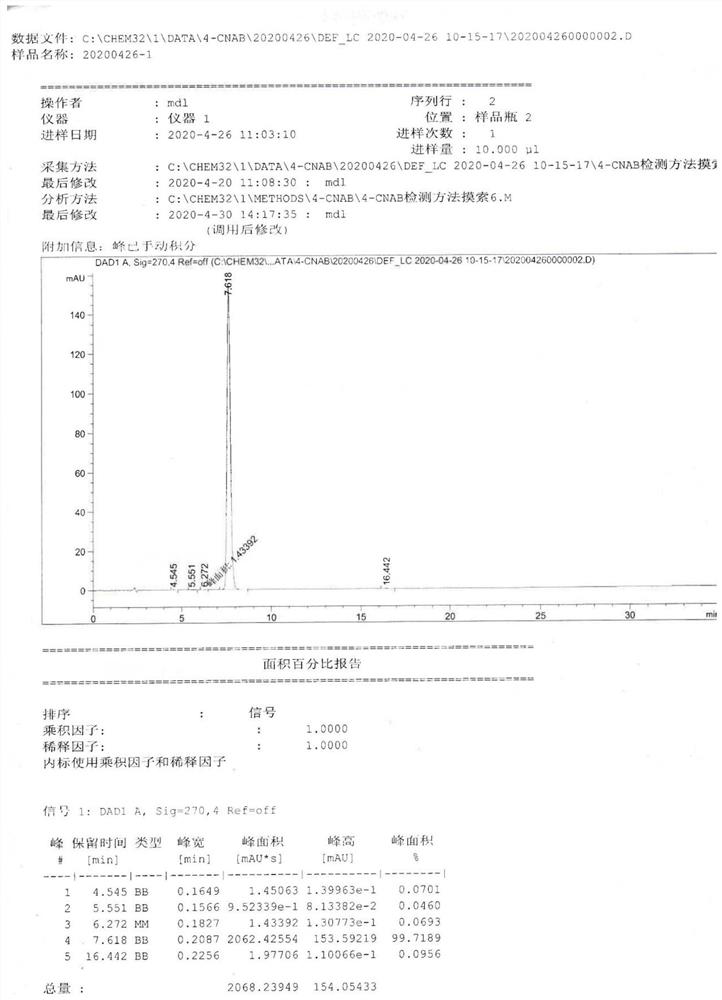
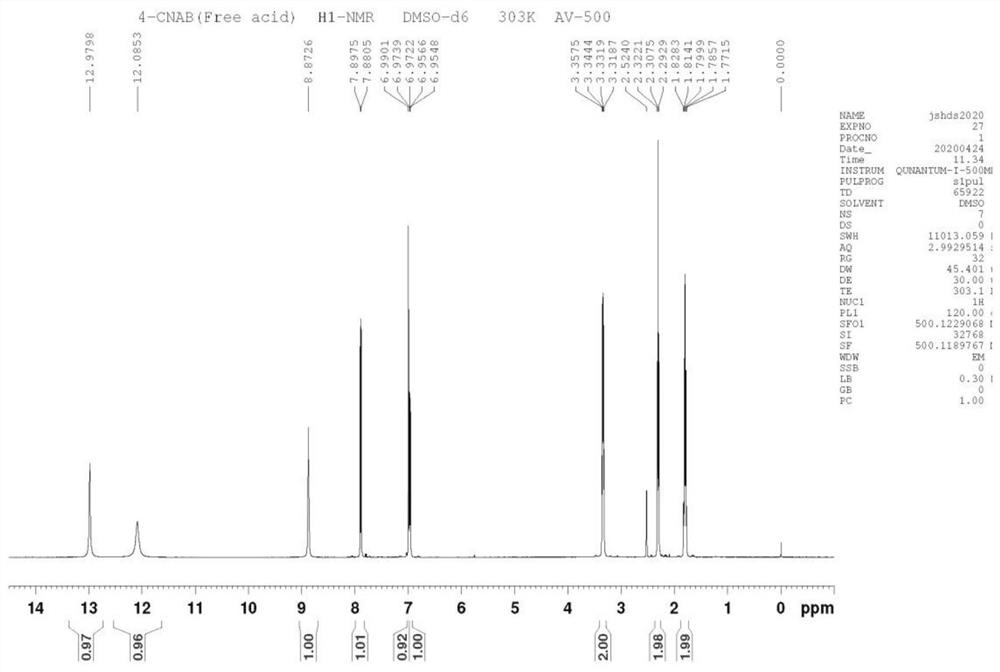
[0052] The reaction solution was moved into a separatory funnel, and an equal volume of 2mol / L dilute hydrochloric acid was added to the reaction solution to adjust the pH value of the reaction solution to 5. After fully shaking, a large amount of solids were precipitated, and suction filtration (diatomaceous earth filter aid) was obtained. cake, and the filtrate was collected; then extracted with dilute ammonia water, pure water, and saturated sodium chloride solution, and the organic phase was concentrated to obtain 74.2 g of interme...
Embodiment 3
[0056] S1: Weigh 200g of 4-chlorosalicylic acid into a 2L reaction bottle, add 800ml of dichloromethane, add 150g of sodium bentonite, slowly add 220g of N,N-carbonyldiimidazole under stirring, a large amount of gas is generated. After the addition, the reaction bottle was moved into a 25°C oil bath, and the stirring was continued at room temperature for 2h. Add 220 g of ethyl 4-aminobutyrate hydrochloride, then dropwise add 220 ml of triethylamine, raise the temperature to 30° C. and stir for 26 h to prepare a reaction solution.
[0057] The reaction solution was moved into a separatory funnel, and an equal volume of 2mol / L dilute hydrochloric acid was added to the reaction solution to adjust the pH value of the reaction solution to 5. After fully shaking, a large amount of solids were precipitated, and suction filtration (diatomaceous earth filter aid) was obtained. Cake, and the filtrate was collected; then extracted with dilute ammonia water, pure water, and saturated sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com