Tumor targeting nanometer micelle, preparation method and application of tumor targeting nanometer micelle as drug carrier
A nanomicelle, tumor targeting technology, applied in the field of biomedicine, can solve the problems of chemoresistant cancer cell clone and distant metastasis, chemotherapy can not achieve therapeutic effect, large side effects, etc., achieves good degradability, suitable for The effect of drug loading and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
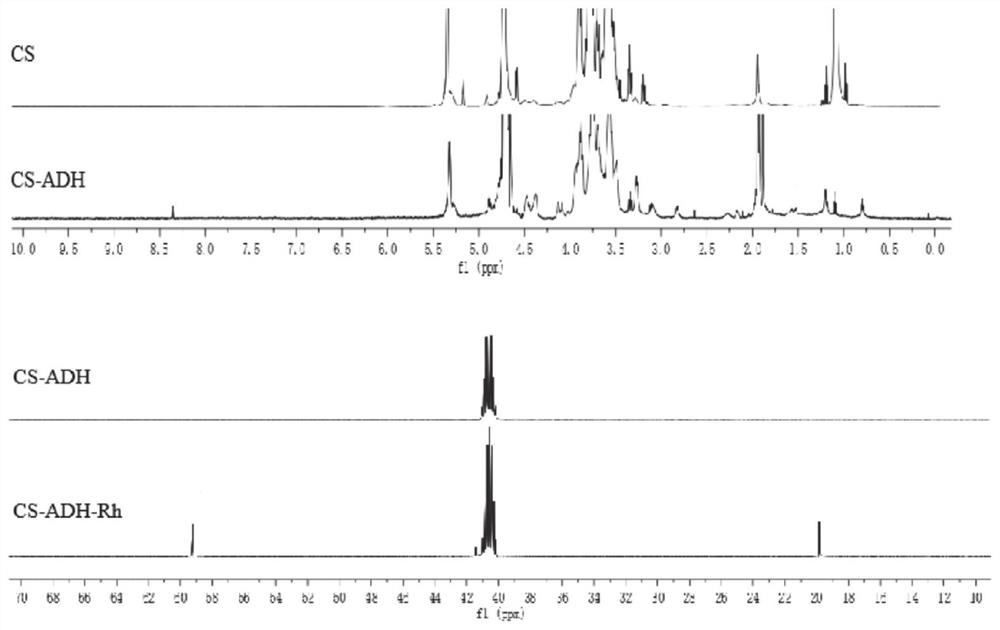
[0079] The synthesis of embodiment 1 chondroitin sulfate-rhein polymer:
[0080] (1) Synthesis of chondroitin sulfate-adipate dihydrazide: Weigh 0.5g chondroitin sulfate and dissolve it in 100mL distilled water, stir to make it fully swell and dissolve, then add 3.52g adipic acid dihydrazide to the solution in turn Hydrazide, 2.0g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 0.8g N-hydroxysuccinimide, reacted at room temperature for 24 hours, dialyzed with distilled water for three days, The intermediate product chondroitin sulfate-adipate dihydrazide was obtained by freeze-drying.
[0081] (2) Weigh 30 mg of rhein and dissolve it in 10 mL of dimethyl sulfoxide, and sequentially add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide equivalent to 5 times the molar amount of rhein Hydrochloride, N-hydroxysuccinimide equivalent to 5 times the molar amount of rhein, stirred at room temperature for 4 hours to activate rhein.
[0082] (3) Synthesis of chondroitin su...
Embodiment 2
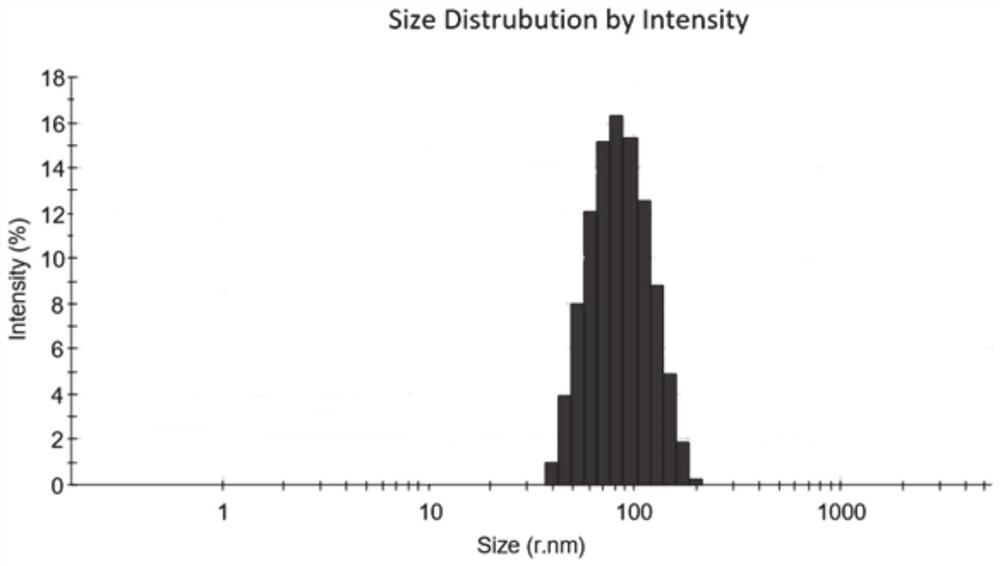
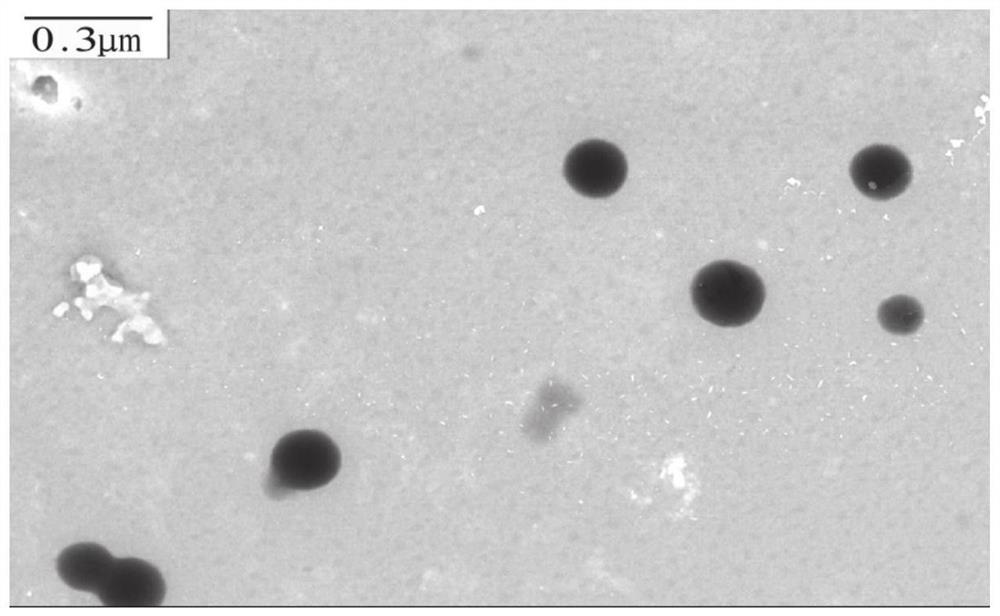
[0086] Example 2 Preparation of cross-linked chondroitin sulfate-rhein-lipoic acid polymer nanomicelles loaded with docetaxel
[0087] (1) 50 mg of chondroitin sulfate-rhein-lipoic acid (prepared in Example 1) was ultrasonically dispersed in 10 mL of deionized water for subsequent use; another 15 mg of docetaxel was weighed and dissolved in 1 mL of methanol, and stirred vigorously Slowly add it dropwise to the above-mentioned chondroitin sulfate-rhein-lipoic acid polymer aqueous solution, and after stirring vigorously at room temperature for 4 hours in the dark, use probe-type ultrasonic treatment at 120W power three times, each time for 4min, pulse on for 2s and stop for 4s, Keep the temperature at 4°C-9°C, then transfer the solution to a dialysis bag and dialyze against water for 12 hours, then add a dithiothreitol solution containing 10% of the ester groups contained in the nanoparticles under strong stirring, and stir at room temperature for 12 hours After that, it was aga...
Embodiment 3
[0089] Example 3 In vitro release test of cross-linked chondroitin sulfate-rhein-lipoic acid nanoparticle micelles loaded with docetaxel
[0090] In this example, DTT is used to simulate the hyperoxic environment in the tumor tissue, and the release rate of the drug is measured by the reverse dynamic dialysis method: take 1 mL of the nano-micelle (prepared in Example 2) solution, and put it in a medium with a molecular weight cut-off of 3500 Daltons In a dialysis bag, use 40ml of phosphate buffered saline (PBS) with a pH of 7.4 containing 0.2% Tween 80 as the release medium, the stirring speed is 100r / min, the temperature is 37±0.5°C, and 1mL of the dialysis medium is taken at a predetermined time And add an equal amount of fresh release medium. After sampling, inject 20 μL of sample for HPLC determination, and calculate the cumulative release percentage, such as Figure 5 shown. The results showed that compared with docetaxel API, the cross-linked chondroitin sulfate-rhein-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com