A kind of preparation method of high-purity nintedanib ethanesulfonate
A high-purity technology for nintedanib ethanesulfonate, which is applied in the field of preparation of high-purity nintedanib ethanesulfonate, can solve problems such as difficult removal, and achieve the effects of improving purity, improving reaction efficiency, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
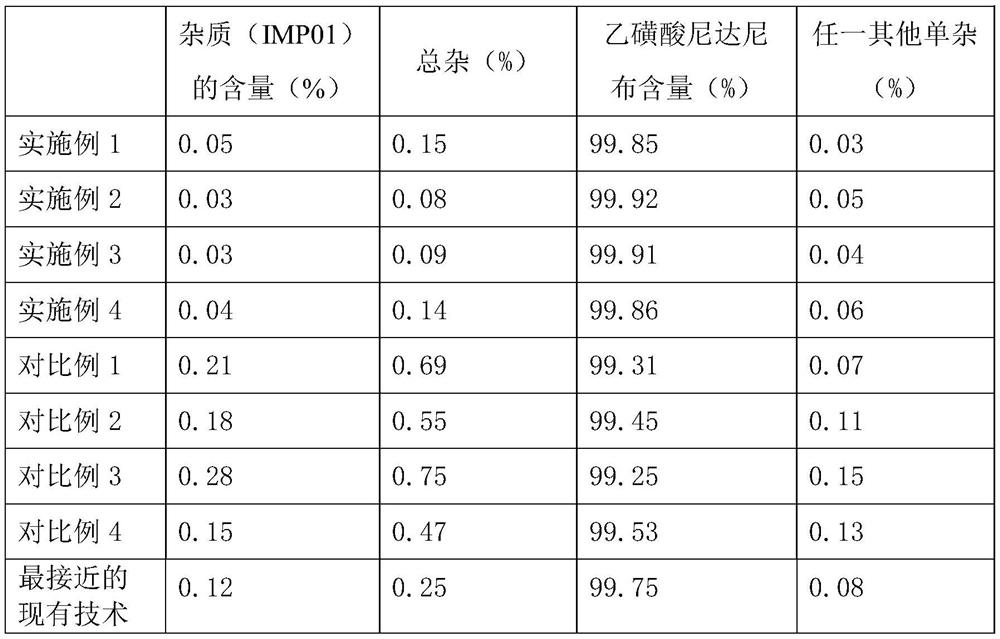
Embodiment 1
[0058] A preparation method for high-purity nintedanib ethanesulfonate, comprising the following steps:
[0059] S1: Preparation of intermediate INT02:
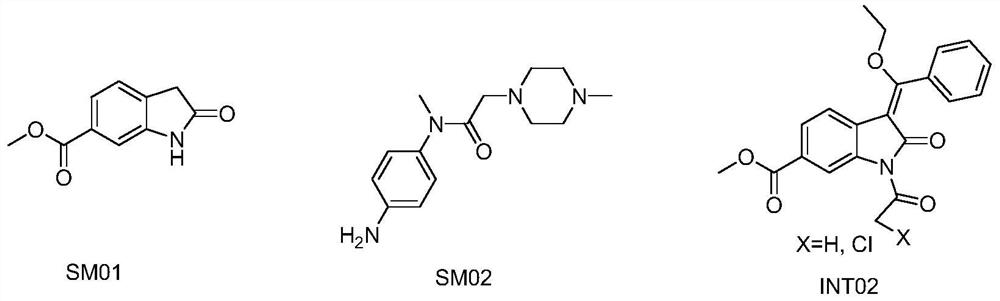
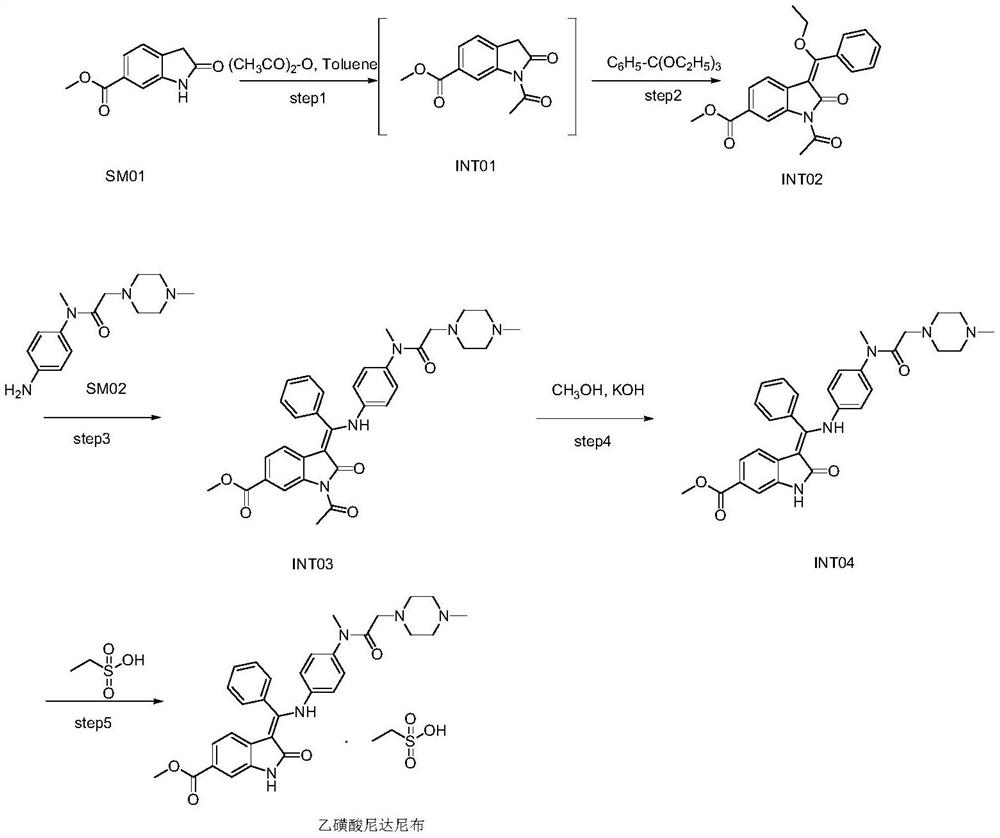
[0060] Add 1730g of toluene, 200g of SM01, 1170g of triethyl orthobenzoate, 1070g of acetic anhydride, 4g of DMAP into the reactor at room temperature, mechanically stir, heat up, and reflux reaction. During the reaction, part of the solvent is evaporated, the temperature is lowered, and the crystal is grown with heat preservation and stirring. Filter, wash the filter cake with toluene, pulverize the wet product, and dry in vacuum to obtain the earthy gray solid product INT02;
[0061] In described step S1, the mass ratio of SM01, triethyl orthobenzoate, acetic anhydride, toluene, DMAP is 1: 5.8: 5.4: 8.7: 0.02, the reflux temperature of reaction is 104-110 ℃, the stirring of reaction Time 7h.
[0062] In step S1, the volume of the distilled solvent is twice the weight of the starting material SM01;
[0063] In step S1, th...
Embodiment 2
[0076] A preparation method for high-purity nintedanib ethanesulfonate, comprising the following steps:
[0077] S1: Preparation of intermediate INT02:
[0078] Add 1000g of toluene, 100g of SM01, 620g of triethyl orthobenzoate, 610g of acetic anhydride, 3g of DMAP into the reactor at room temperature, mechanically stir, heat up, and reflux reaction. During the reaction, part of the solvent is evaporated, the temperature is lowered, and the crystal is grown with heat preservation and stirring. Filter, wash the filter cake with toluene, pulverize the wet product, and dry in vacuum to obtain the earthy gray solid product INT02;
[0079] In described step S1, the mass ratio of SM01, triethyl orthobenzoate, acetic anhydride, toluene, DMAP is 1: 6.2: 6.1: 10: 0.03, the reflux temperature of reaction is 106-114 ℃, the stirring of reaction Time 6h.
[0080] In step S1, the volume of the distilled solvent is 3 times the weight of the starting material SM01;
[0081] In step S1, the...
Embodiment 3
[0094] A preparation method for high-purity nintedanib ethanesulfonate, comprising the following steps:
[0095] S1: Preparation of intermediate INT02:
[0096] Add 1200g of toluene, 100g of SM01, 520g of triethyl orthobenzoate, 720g of acetic anhydride, 4g of DMAP into the reactor at room temperature, mechanically stir, heat up, and reflux reaction. During the reaction, part of the solvent is evaporated, the temperature is lowered, and the crystal is grown with heat preservation and stirring. Filter, wash the filter cake with toluene, pulverize the wet product, and dry in vacuum to obtain the earthy gray solid product INT02;
[0097] In described step S1, the mass ratio of SM01, triethyl orthobenzoate, acetic anhydride, toluene, DMAP is 1: 5.2: 7.2: 12: 0.04, the reflux temperature of reaction is 104-114 ℃, the stirring of reaction Time 8h.
[0098] In step S1, the volume of the distilled solvent is 4 times the weight of the starting material SM01;
[0099] In step S1, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com