Method for promoting over-expression of TSP4 in mesenchymal stem cells, and preparation and application of mesenchymal stem cells
A mesenchymal stem cell and overexpression technology, which is applied in the field of lentivirus-infected mesenchymal stem cell preparations, can solve the problems of slow formation of new blood vessels, low number of mesenchymal stem cells, poor treatment effect, etc., so as to improve the efficiency of angiogenesis and promote the Effects of paracrine function, enhancement of self-renewal and multi-directional differentiation and hyperproliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] A preparation method of Plv-Easy-GFP-TSP4 lentivirus.
[0097] (1) Using the snapgene software, design corresponding primers according to the sequence of the coding region (CDS region) of the plasmid pCMV6-Thbs4 gene: upstream: 5'CGGGATCCATGCCGGCCCCAC3'; downstream: 5'CCGCTCGAGATTATCCAAGCGGTC3'.
[0098] (2) Perform polymerase chain reaction amplification on the upper primers in the system shown in Table 4 below to obtain oligonucleotide chains.
[0099] Table 4
[0100]
[0101]
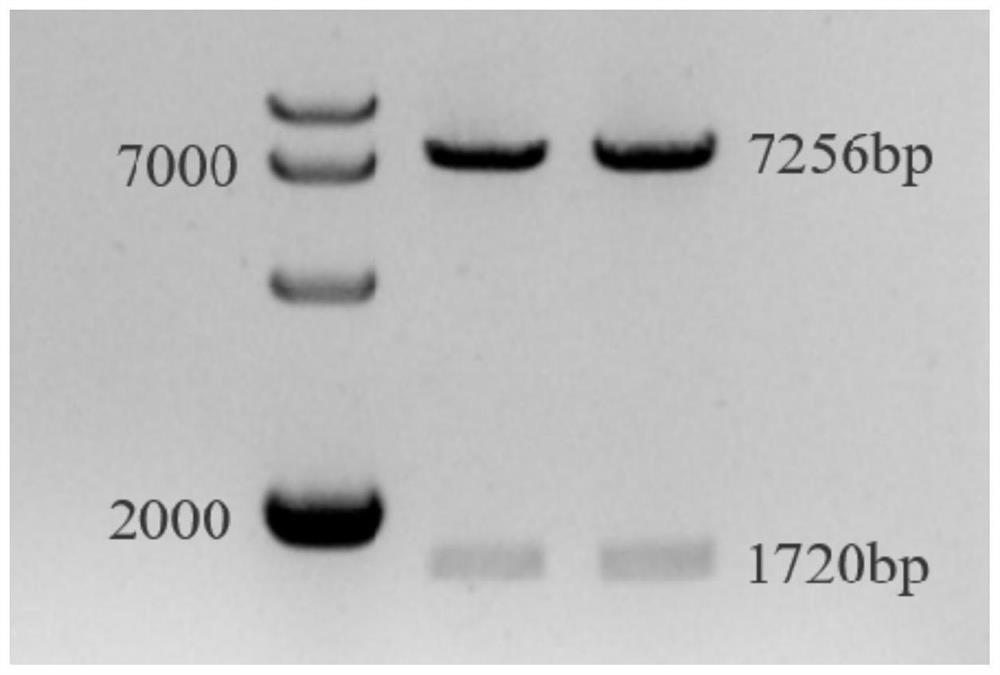
[0102] Wherein, the DNA template is the pCMV6-Thbs4 plasmid, and the extension rate is 15-30s / kb. After the PCR amplification reaction is completed, the gel is recovered and purified to obtain a purified DNA solution.
[0103] (3) In the system shown in Table 5 below, the pENTR11 plasmid vector was double-digested with XhoI and BamHI to obtain the pENTR11 plasmid vector with cohesive ends.
[0104] table 5
[0105]
[0106] (4) In the ligation reaction system of Table 6 below, the...
Embodiment 2
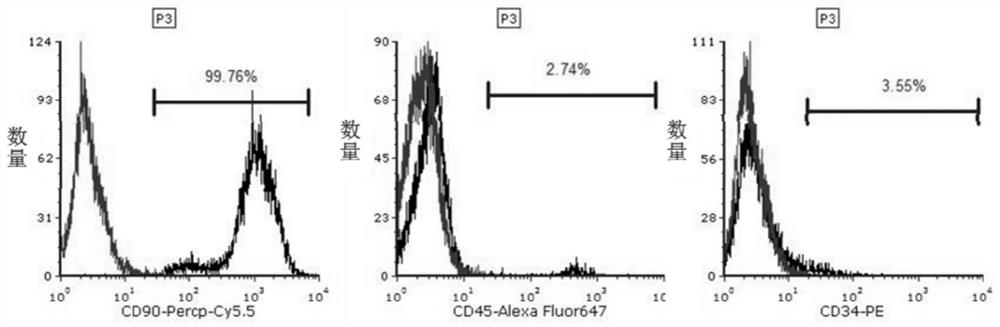
[0114] A method for culturing bone marrow mesenchymal stem cells (BMSCs).
[0115] (1) SD rats (50±10) g were killed by neck dislocation, soaked in 75% alcohol for 10 minutes.
[0116] (2) Isolate the rat femur under sterile conditions, cut off the femoral metaphysis, use a 5ml syringe to absorb DMEM / F12 complete medium (DMEM / F12+20%FBS+1% double antibody) and flush out the bone marrow to 10cm 2 In the cell culture dish, the cell suspension was made by blowing with a pipette gun.
[0117] (3) Transfer the above cell suspension into a 15ml centrifuge tube, label it, centrifuge at 800rpm*10min, and discard the supernatant. After resuspending the cells in DMEM / F12 complete medium, move the cell suspension to 25cm 2 culture flask at 37°C 95% CO 2 The cells were cultured in an incubator, and the medium was changed after 48 hours.
[0118] (4) When the cell density reaches above 80%, digest the cells with 0.25% trypsin and spread to 75cm 2 Cell culture flasks continued to grow....
Embodiment 3
[0123] A method for infecting bone marrow mesenchymal stem cells (TSP4-BMSC) with Plv-Easy-GFP-TSP4 lentivirus.
[0124] (1) Inoculate the bone marrow mesenchymal stem cells into a cell culture flask, and culture them in the medium of DMEM / F12+10% FBS+1% double antibody in a cell culture incubator at 37°C until the bone marrow mesenchyme The density of the stem cells is 80%, and the first culture product is obtained;
[0125] (2) replacing the medium of the first culture product with a medium comprising DMEM / F-12 and 5% FBS, and culturing at 37° C. for 2 hours to obtain a second culture product;
[0126] (3) replacing the medium of the second culture product with a lentivirus medium, and culturing at 37° C. for 6 hours to obtain a third culture product;
[0127] (4) The medium of the third culture product was replaced with a medium comprising DMEM / F-12, 5% FBS and 1‰GM, and cultured for 72 hours to obtain lentivirus-infected bone marrow mesenchymal stem cells (TSP4-BMSC ). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com