Injectable composition comprising hyaluronidase for removing local fat
An injectable composition, hyaluronidase technology, applied in the field of injectable composition, can solve the problem of unsatisfactory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0097] Preparation of injectable composition
[0098] The composition and composition ratio of the injectable composition are described in Table 1. The composition containing hyaluronidase in an amount of less than 300 IU is designated as Comparative Example 1, and the composition containing hyaluronidase in an amount of more than 600 IU is designated as Comparative Example 1. Designated as Comparative Example 2, a composition containing 300 IU of hyaluronidase was designated as Comparative Example 3, and a composition according to the present invention was designated as an Example.
[0099] [Table 1]
[0100] ingredient Comparative example 1 Comparative example 2 Comparative example 3 Example Hyaluronidase25,000IU65,000IU30,000IU30,000IU Lidocaine 21.0mg 21.0mg 21.0mg 21.0mg Pheniramine 1.2mg 1.2mg 1.2mg 1.2mg L-carnitine 66.0mg 66.0mg 66.0mg 66.0mg Vitamin C 60.0mg 60.0mg 60.0mg 60.0mg Adrenaline 5.0mg Choline alfoscerate 40.0mg Sodium bicarbonate 20.0mg Salt solution ...
experiment Embodiment 1
[0103] Lipolysis of the injectable composition of the present invention
[0104] The changes in waist circumference after administration of the injectable compositions of the Examples and the injectable compositions of Comparative Examples 1 to 3 and the changes in waist circumference after the endomology treatment were measured and shown in Table 2.
[0105] The treated subjects were a total of 40 abdominal obese patients (20 males, 20 females) with a BMI of 25 or greater selected from adult outpatients aged 20 years or older.
[0106] In the abdominal area between the ribs and the pubic bone, 50 points were injected with 1 cc of the injectable composition, and the 50 points were arranged so that they were 1 cm apart from the navel. Repeat the injection every week for four weeks, moving the injection point each time. The reduction in waist circumference was measured 1 week after the completion of the treatment, and then measured again 1 month after the completion of the treatment. ...
experiment Embodiment 2
[0117] Evaluation of the side effects of the injectable composition of the present invention
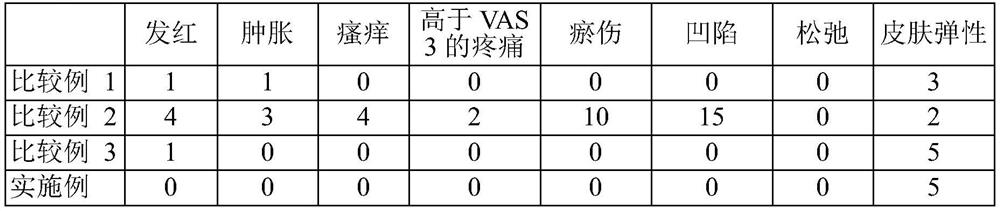
[0118] The injectable compositions of the Examples and the injectable compositions of Comparative Examples 1-3 were administered in the same manner as in Experimental Example 1. The side effects observed in each patient in the clinical trial are shown in Table 3.
[0119] [table 3]
[0120]
[0121] *Skin elasticity is determined by tactile evaluation using a 5-point scale: 1: very poor; 2: poor; 3: fair; 4: good; 5: very good.
[0122] As shown in Table 3, compared with Comparative Examples 1 to 3, the injectable composition according to the examples of the present invention showed significantly reduced allergic redness, swelling, itching and pain.
[0123] These effects are believed to be due to the use of local anesthetics, antihistamines, epinephrine, methacholine and sodium bicarbonate, and low-dose hyaluronidase.
[0124] Overall, it is believed that the above-mentioned effects are due to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com