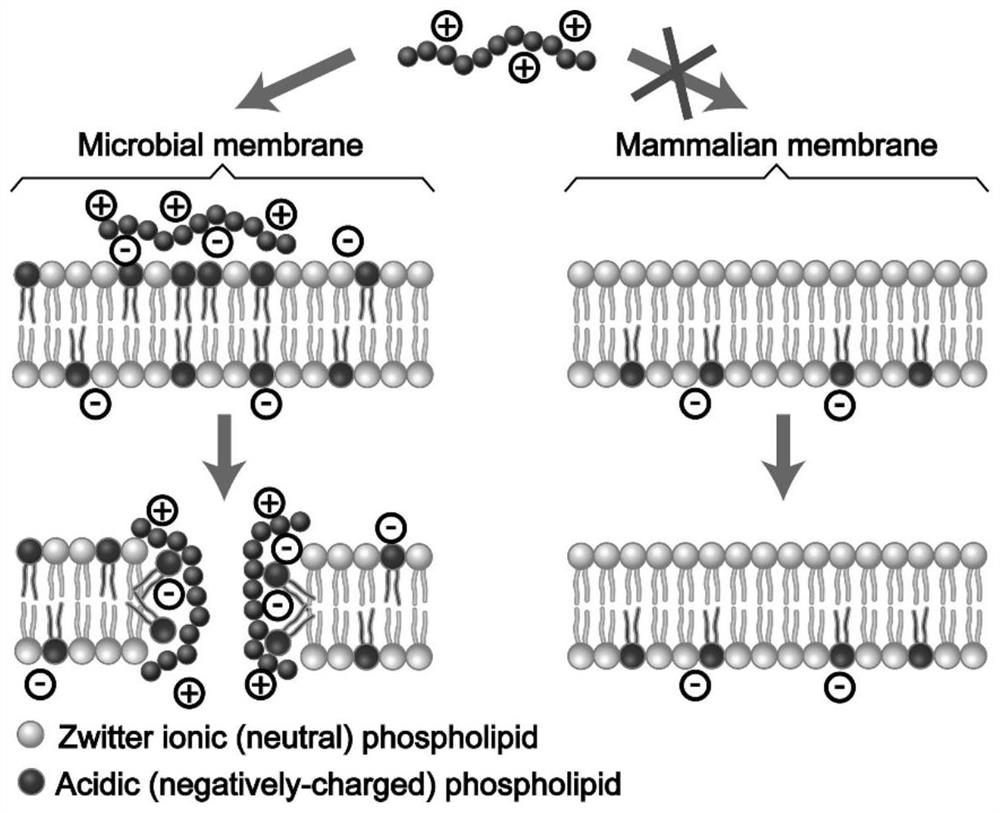
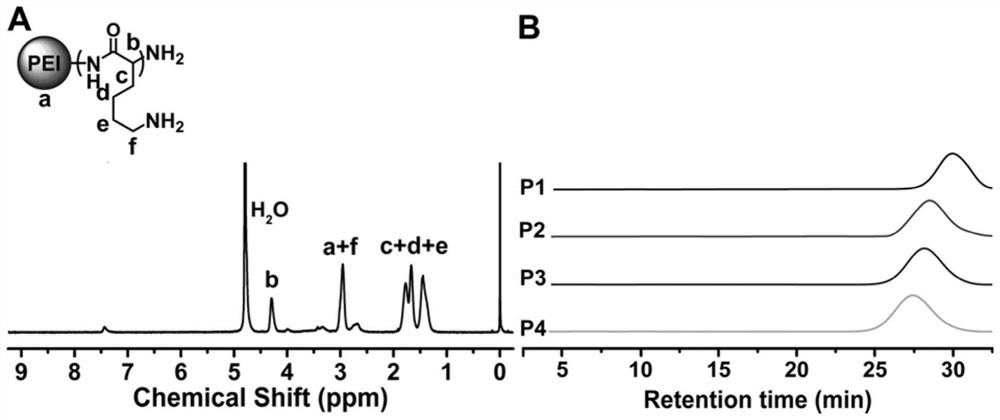
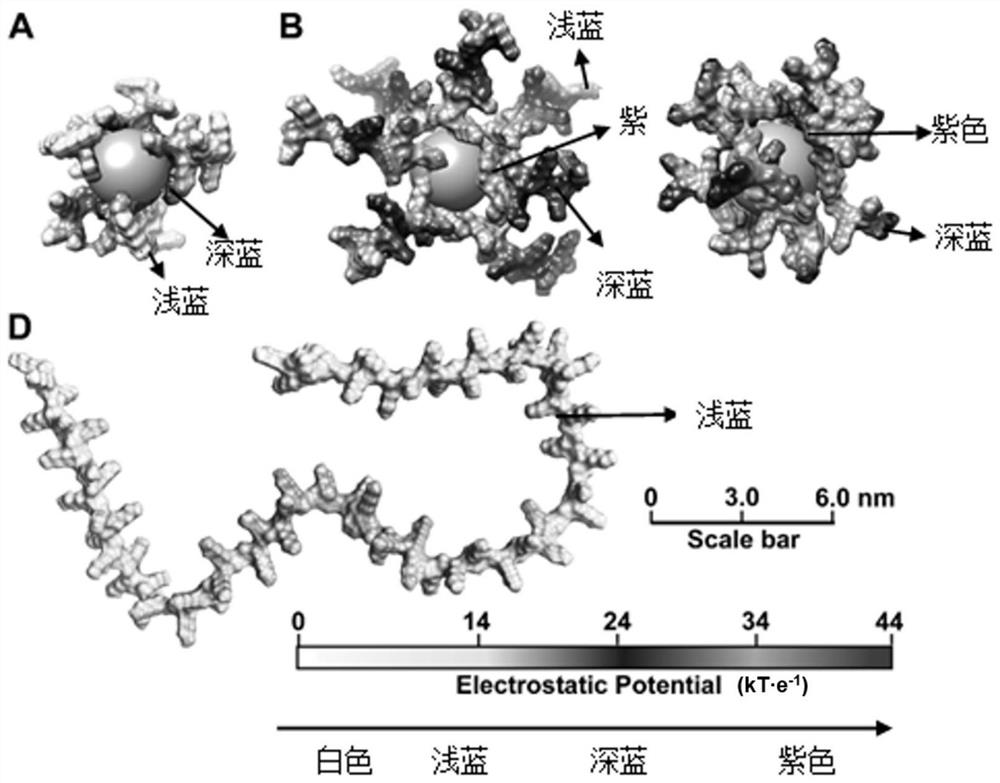
Branched antibacterial polyamino acid, and preparation method and application thereof
A polyamino acid and polylysine technology, applied in the field of medicine, can solve the problems of increasing charge density, hindering the development and limitation of antimicrobial peptides or polymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The invention also provides a preparation method of the branched antibacterial polyamino acid, including a liquid phase synthesis method (amino acid N-carboxy-anhydride (NCA) ring-opening polymerization method) and a solid phase synthesis method. The branched antibacterial polyamino acid synthesized by the liquid phase synthesis method (amino acid N-carboxyl-anhydride ring-opening polymerization method) has the advantages of simple operation, high yield, low production cost, no racemization, and easy access to high molecular polyamino acid And many other advantages, but the molecular weight distribution of the synthesized polymer is relatively wide. The sequence and molecular weight of the polypeptide can be precisely controlled by solid-phase synthesis, but the synthesis time is long, the cost is high, and the sequence directly synthesized is relatively short. Therefore, although the antibacterial polyamino acids synthesized by the two methods have the characteristics ...
Embodiment 1
[0057] The liquid phase synthesis method of embodiment 1 star-shaped PLL antibacterial polyamino acid (PEI-g-PLL) (amino acid N-carboxyl-ring-opening polymerization method of acid anhydride in the ring)
[0058] 1. Synthesis of star-shaped poly-L-lysine (PLL)
[0059] (1) Synthesis of amino acid N-carboxy-anhydride (NCA)
[0060] Weigh an appropriate amount of ε-benzyloxycarbonyl-L-lysine, activated carbon and triphosgene (BTC) into anhydrous ethyl acetate (EA) and heat to reflux, and filter with suction to obtain the filtrate containing the crude NCA product. The reaction scheme is as follows:
[0061]
[0062] Freeze the filtrate to below -18°C, wash with 4°C saturated sodium bicarbonate solution and saturated sodium chloride solution respectively to remove residual hydrochloric acid and triphosgene in the system. Finally, the product passes through the process of crystallization and recrystallization in ethyl acetate-petroleum ether mixed solvent to obtain high-purity N...
Embodiment 2
[0072] The solid-phase synthesis method of embodiment 2 star-shaped PLL antibacterial polyamino acids
[0073] 1. Synthesis of star PLL
[0074] (1) Synthesis of dendritic PLL core
[0075]Remove the Fmoc protecting group of Rink Amide-MBHA Resin resin with 20% piperidine in DMF solution, and add N,N'-bisfluorenylmethoxycarbonyl-L-lysine (Fmoc-Lys(Fmoc)-OH) , using DMF as a solvent, 1-hydroxybenzotriazole (HOBt) and N, N'-diisopropylcarbodiimide (DIC) as a condensing agent for coupling reaction, and then using DMF containing 20% piperidine The solution removes the Fmoc protecting group to obtain a generation of dendritic poly-L-lysine. Then, through repeated coupling and deprotection processes, the second-generation dendritic PLL with 4 amino groups and the third-generation dendritic poly-PLL with 8 amino groups are obtained as the core of the star-shaped PLL. The synthetic route is as follows:
[0076]
[0077] (2) Synthesis and characterization of star PLL
[0078] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com