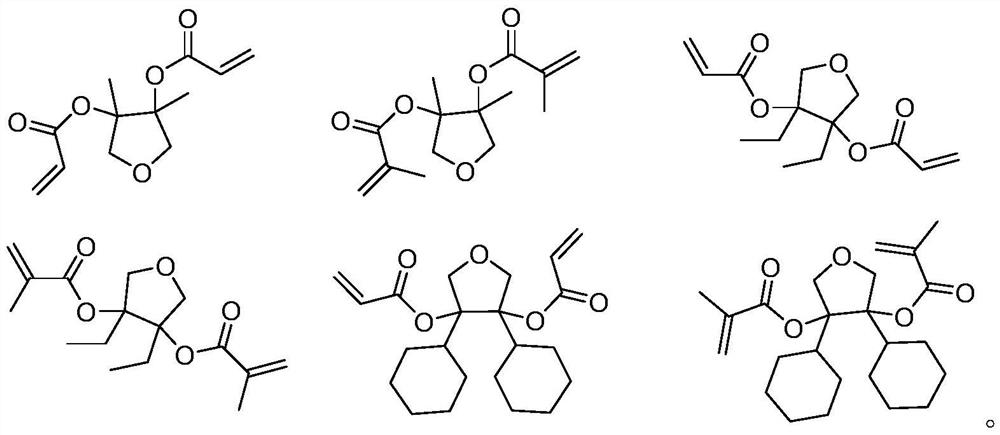
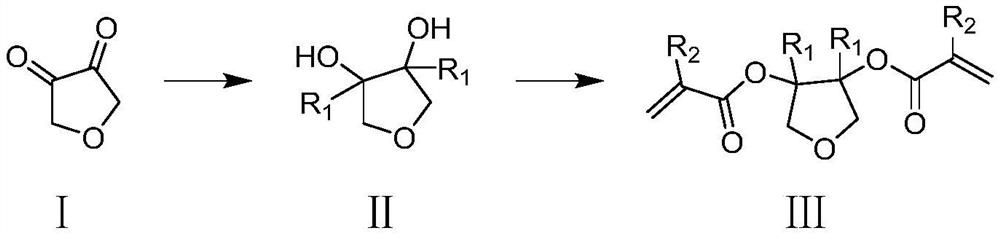
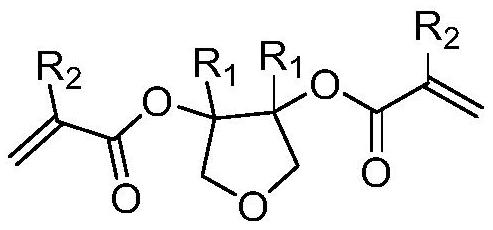
Degradable photoresist resin monomer synthesized from furandione and synthesis method thereof
A technology of resin monomer and furandione, applied in the field of resin monomer and its synthesis, can solve the problems of insufficient resolution and weak etching resistance, achieve good solubility, good etching resistance, and improve edge roughness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] The first step: a. Preparation of methyl Grignard reagent: adding magnesium chips (4.9g, 202mmol) to anhydrous ether (15mL), then adding an iodine tablet, and dissolving methyl bromide (19g, 200mmol) Prepare a solution in diethyl ether (25mL). Under nitrogen protection, first add methyl bromide diethyl ether solution (6mL) to the above reaction solution. After a few minutes, the reaction solution boils slightly and the color of iodine disappears. Continue to drop Add the remaining ether solution of methyl bromide, add ether (20mL), raise the temperature and keep boiling slightly, and reflux for half an hour; b. Synthesis of intermediate 1-2: under nitrogen protection, the prepared methyl Grignard reagent Cool in water, add furan-3,4(2H, 5H)-diketone (10.0g, 55mmol) dropwise in diethyl ether (20mL) solution under stirring, control the rate of addition, keep the reaction solution slightly boiling, after the dropwise addition, at 25 Stirring was continued for h...
Embodiment 2
[0030]
[0031] The first step: the operation steps and raw material dosage are the same as the first step reaction in Example 1, and the reaction obtains compound 2-2 (11.5g, 87mmol, 87.1%);
[0032] The second step: the operation steps are the same as the second step reaction of Example 1, wherein the reactants and the charging amount: intermediate 1-2 (11.3g, 86mmol) is replaced by intermediate 2-2 (11.5g, 87mmol), propylene Acyl chloride (15.5g, 171mmol) was replaced by methacryloyl chloride (18.2g, 174mmol), triethylamine (34.6g, 342mmol) was replaced by triethylamine (35.3g, 349mmol), and compound 2-3 (19.3g, 72 mmol, 82.7%).
Embodiment 3
[0034]
[0035] The first step: the operation steps are the same as the first step of Example 1, wherein methyl bromide (19g, 200mmol) is changed to ethyl bromide (21.8g, 200mmol), to obtain compound 3-2 (13.5g, 84mmol, 84.3% );
[0036] The second step: the operation steps are the same as the second step reaction of Example 1, wherein reactants and charging amount: intermediate 1-2 (11.3g, 86mmol) is replaced by intermediate 3-2 (13.5g, 84mmol), propylene Acyl chloride (15.5g, 171mmol) was replaced by acryloyl chloride (15.3g, 169mmol), triethylamine (34.6g, 342mmol) was replaced by triethylamine (34.2g, 338mmol), and compound 3-3 (19.5g, 73mmol, 86.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com