Crystal form of alkynyl-containing compound salt, preparation method and application thereof
A crystal form, ethynyl technology, applied in the preparation of organic compounds, separation/purification of carboxylic acid compounds, preparation of carboxylate, etc., can solve the problems of polycrystalline compounds and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
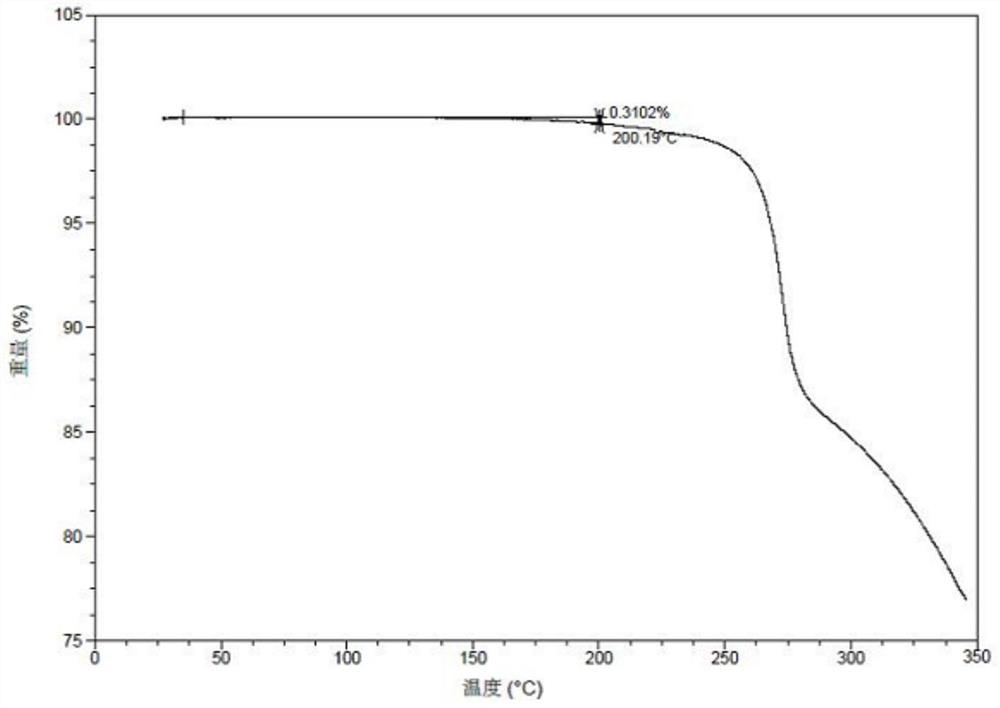
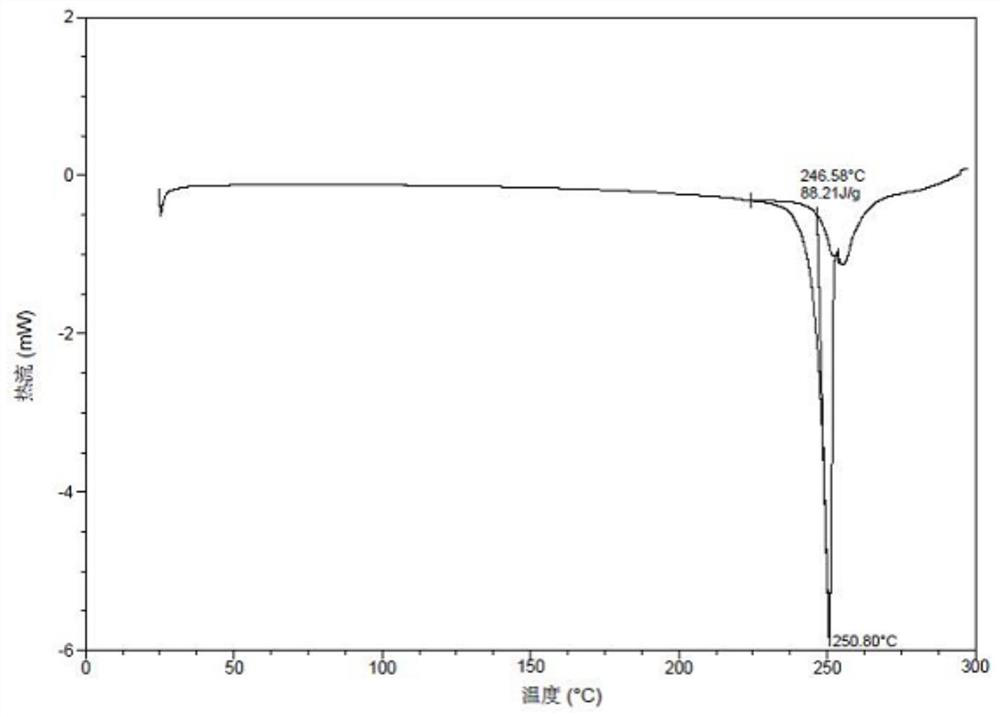
[0079] Example 1: Preparation of Form II
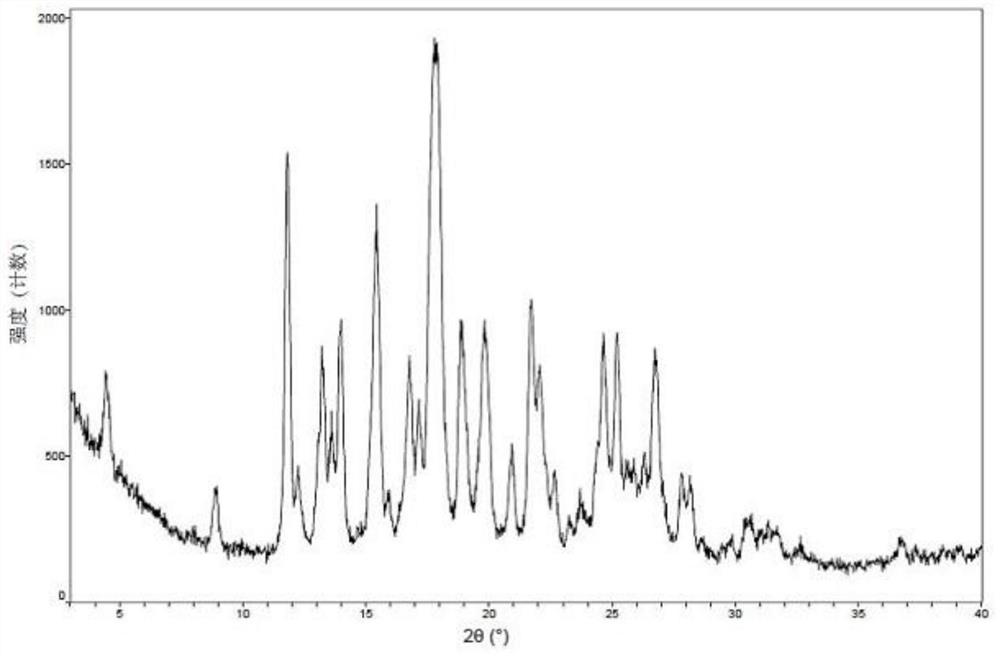
[0080] 40mg 3-((1H-pyrazole[3,4-b]pyridine-5-substituted)ethynyl)-4-methyl-N-(4-((4-methylpiperazine-1-substituted) (Methyl)-3-(trifluoromethyl)phenyl)benzamide was added to 0.5mL of methanol and dichloromethane (the volume ratio of methanol and dichloromethane is 1:1), stirred at room temperature, and 1 equivalent was added. Maric acid immediately appeared as a solid, continue to stir overnight, filter, and vacuum dry at 50°C overnight to obtain crystal form II. The XPRD pattern of Form II is as follows figure 1 Shown. In the X-ray powder diffraction pattern expressed in 2θ angles, the 2θ values are shown in Table 1;
[0081] Table 1
[0082] Diffraction angle Relative Strength d valueDiffraction angle Relative Strength d value (2θ°)(%) (Angstrom) (2θ°)(%) (Angstrom) 4.46141.419.7933324.3527.13.6525 8.904209.92424.65248.13.60846 11.81780.67.4831725.19848.23.5315 12.24424.37.2232425.8825.73.43995 13.25145.86.6764526.30126.83.38583...
Embodiment 2
[0085] Example 2: 3-((1H-pyrazole[3,4-b]pyridine-5-substituted)ethynyl)-4-methyl-N-(4-((4-methylpiperazine-1- (Substituted)methyl)-3-(trifluoromethyl)phenyl)benzamide monohydrochloride form III
[0086] 40mg of 3-((1H-pyrazole[3,4-b]pyridine-5-substituted)ethynyl)-4-methyl-N-(4-((4-methylpiperazine-1-substituted)methyl (Methyl)-3-(trifluoromethyl)phenyl)benzamide was added to 0.2mL of methanol and dichloromethane (the volume ratio of methanol to dichloromethane is 1:1), mixed into the system, stirred at room temperature, and 1.05 equivalent was added The hydrochloric acid, the solid was completely dissolved, ethyl acetate was added, the stirring was continued for 0.5 h, the solid was precipitated, the stirring was continued for 4 h, filtered, and dried under vacuum at 50° C. overnight to obtain crystal form III.
[0087] The X-ray powder diffraction pattern of Form III expressed at 2θ angles, the 2θ values are shown in Table 2;
[0088] Table 2 2θ Values of Form III
[0089] ...
Embodiment 3
[0092] Example 3: Preparation of Form IV
[0093] 40mg of 3-((1H-pyrazole[3,4-b]pyridine-5-substituted)ethynyl)-4-methyl-N-(4-((4-methylpiperazine-1-substituted)methyl Base)-3-(trifluoromethyl)phenyl)benzamide was added to 0.4mL of methanol and dichloromethane (the volume ratio of methanol to dichloromethane is 1:1), mixed into the system, stirred at room temperature, and 2.1 equivalents were added The hydrochloric acid, a solid precipitated out, continued to stir for 1-2h, filtered, and dried under vacuum at 50°C overnight to obtain crystal form IV.
[0094] In the X-ray powder diffraction diagram of Form IV expressed at 2θ angles, the 2θ values are shown in Table 3;
[0095] Table 3 2θ Values of Form IV
[0096] Diffraction angle Relative Strength d valueDiffraction angle Relative Strength d value (2θ°)(%) (Angstrom) (2θ°)(%) (Angstrom) 4.29310020.5655316.62956.35.3268 5.14779.217.1562116.79758.55.27394 6.24287.414.1490617.22479.75.14416 9.02141.59.7952218.50148.84.79187 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com