Nifedipine sustained-release preparation and preparation thereof
A technology of nifedipine and sustained-release preparations, applied in the field of medicine, can solve the problems of unsatisfactory long-term stability, mixed quality and low bioavailability, and achieve the advantages of reducing the number of medications, taking them conveniently, and uniformly dispersing the dosage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
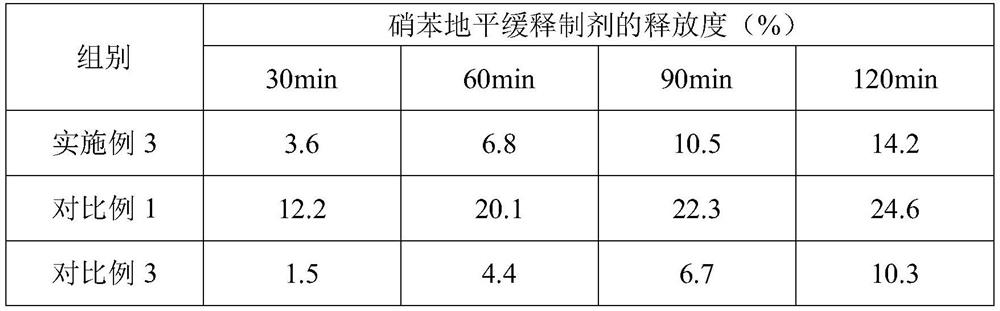
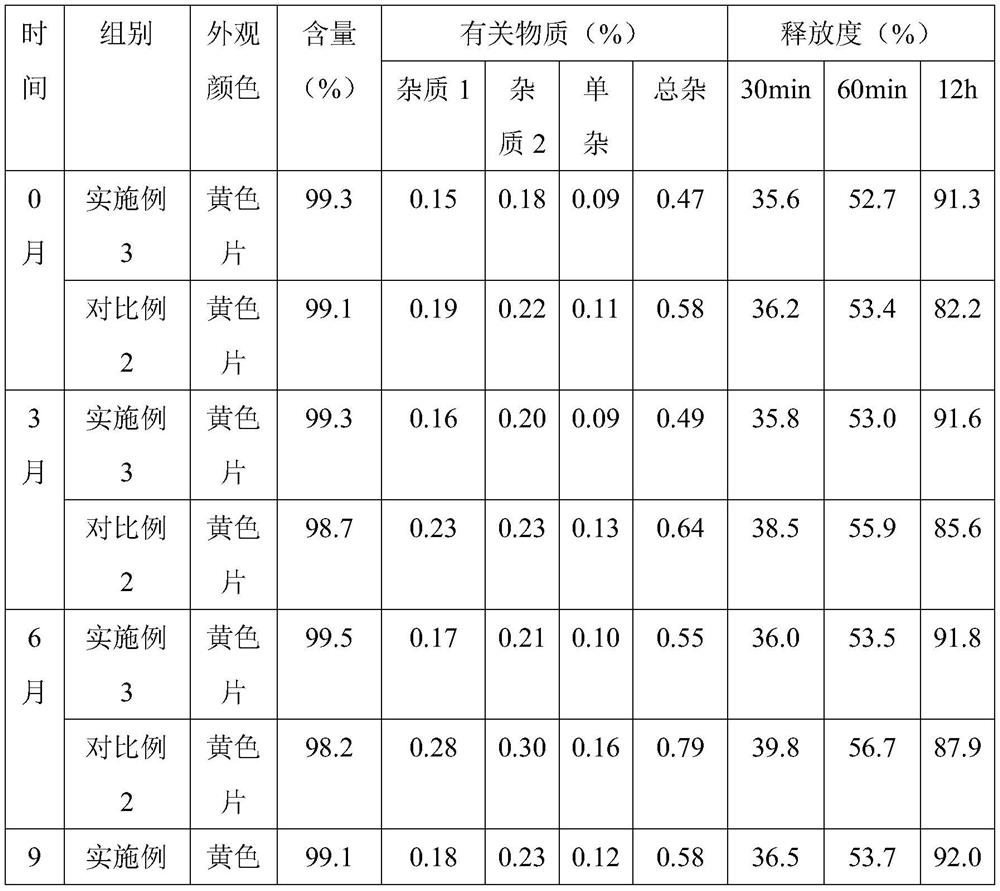
[0035] Embodiment 1, a kind of nifedipine sustained-release preparation
[0036] The nifedipine sustained-release preparation comprises the following components and parts by mass thereof:
[0037] 15 parts of nifedipine, 15 parts of slow-release agent, 7 parts of binder, 50 parts of filler, 0.3 part of glidant, 3 parts of disintegrant, 0.2 part of magnesium stearate; Sodium, crospovidone and polyacrylic acid are composed of 10:6:3 by mass ratio; the binder is composed of polyvinyl alcohol and polyvinylpyrrolidone by 5:2 by mass ratio; the filler is composed of microcrystalline cellulose and The compressible starch is composed of a mass ratio of 5:2; the glidant is silicon dioxide; and the disintegrant is crospovidone.
[0038] The preparation method of described nifedipine sustained-release preparation, the steps are as follows:
[0039] S1 Grinding nifedipine to a particle size D90 of 10 μm to obtain nifedipine powder;
[0040] S2 respectively pulverize the sustained-relea...
Embodiment 2
[0045] Embodiment 2, a kind of nifedipine sustained-release preparation
[0046] The nifedipine sustained-release preparation comprises the following components and parts by mass thereof:
[0047] 20 parts of nifedipine, 25 parts of slow-release agent, 10 parts of binder, 70 parts of filler, 0.5 part of glidant, 5 parts of disintegrant, 0.6 part of magnesium stearate; Sodium, crospovidone and polyacrylic acid are composed of 15:3:1 by mass ratio; the binder is composed of polyvinyl alcohol and polyvinylpyrrolidone by 8:1 by mass ratio; the filler is composed of compressible starch, The mass ratio of mannitol and lactose is 10:5:3; the glidant is talc; the disintegrant is composed of sodium carboxymethyl starch and sodium lauryl starch in a mass ratio of 1:5.
[0048] The preparation method of described nifedipine sustained-release preparation, the steps are as follows:
[0049]S1 Grinding nifedipine to a particle size D90 of 50 μm to obtain nifedipine powder;
[0050] S2 re...
Embodiment 3
[0055] Embodiment 3, a kind of nifedipine sustained-release preparation
[0056] The nifedipine sustained-release preparation comprises the following components and parts by mass thereof:
[0057] 18 parts of nifedipine, 22 parts of sustained-release agent, 8 parts of binder, 62 parts of filler, 0.4 part of glidant, 4 parts of disintegrant, 0.4 part of magnesium stearate; Sodium, crospovidone and polyacrylic acid are composed of 12:5:2 by mass ratio; the binder is composed of polyvinyl alcohol and polyvinylpyrrolidone by 6:1 by mass ratio; the filler is composed of microcrystalline cellulose, Compressible starch and mannitol are formed in a mass ratio of 7:4:2; the glidant is silicon dioxide; the disintegrant is composed of carboxymethylcellulose calcium and sodium lauryl starch in a mass ratio of 9 :2 composition.
[0058] The preparation method of described nifedipine sustained-release preparation, the steps are as follows:
[0059] S1 Grinding nifedipine to a particle si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com