Preparation method of R-1-(naphthalene-1-yl) ethanol
A technology for ethanol and naphthoethyl ketone, which is applied in the field of preparation of R-1-ethanol, can solve the problem that the chiral purity of R-configuration alcohol needs to be further improved, and achieves the solution of metal residual risk, high recovery rate and reduction of synthesis cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] Embodiments of the present invention also provide a synthetic method of R-1-(naphthalen-1-yl)ethylamine, comprising the following steps:
[0063] Synthesize R-1-(naphthalene-1-yl)ethanol according to the synthetic method described above;
[0064] The R-1-(naphthalene-1-yl)ethanol is subjected to sulfonylation and aminolysis.
[0065] R-1-(naphthalen-1-yl)ethylamine is an important intermediate of the chiral drug cinacalcet. Specifically, the reaction process of the synthetic method of above-mentioned R-1-(naphthalene-1-yl)ethylamine is as follows:
[0066]
[0067] The present invention also provides a synthetic method of cinacalcet, the steps of which include: synthesizing R-1-(naphthalen-1-yl)ethylamine by the aforementioned synthetic method.
[0068] Cinacalcet is the first drug in a new class of compounds known as calcimimetics, which activate calcium receptors in the parathyroid glands, thereby reducing the secretion of parathyroid hormone (PTH), thereby Effe...
Embodiment 1
[0073] This embodiment is a synthetic method of R-1-(naphthalene-1-yl)ethanol, the steps are as follows:
[0074] (1) Feed argon into the 5L autoclave to replace the air to form an argon atmosphere, add 100g of raw material 1-naphthyl ethyl ketone from the feed port of the autoclave, then add 1L of toluene to fully dissolve the raw material, continue Introduce argon gas for bubbling and degassing, continue bubbling for 1 hour, and degassing is completed.
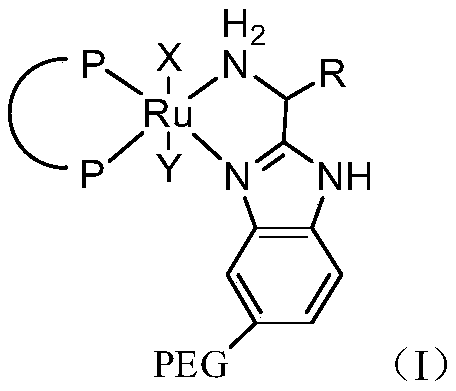
[0075] (2) Under argon atmosphere, add 0.05g catalyst (wherein, the degree of polymerization of PEG n=1000) from the feed port of the autoclave, and finally add 10g potassium ethylate, after the feed is completed, quickly close the feed port.
[0076] (3) Replace the argon in the autoclave with hydrogen, then slowly feed hydrogen until the pressure in the autoclave is 3 atm, close the inflation valve; stir and react rapidly at a temperature of 40°C. During the reaction process, if the pressure drops, the hydrogen gas is sup...
Embodiment 2
[0080] This embodiment is a synthetic method of R-1-(naphthalene-1-yl)ethanol, the steps are as follows:
[0081] (1) Feed argon into the 5L autoclave to replace the air to form an argon atmosphere, add 100g of raw material 1-naphthyl ethyl ketone from the feed port of the autoclave, then add 1L of toluene to fully dissolve the raw material, continue Introduce argon gas for bubbling and degassing, continue bubbling for 1 hour, and degassing is completed.
[0082] (2) Under argon atmosphere, add 0.05g catalyst (wherein, the polymerization degree n=800 of PEG) from the feed port of the autoclave, and finally add 10g potassium ethylate, and close the feed port quickly after the feed is completed.
[0083] (3) Replace the argon in the autoclave with hydrogen, then slowly feed hydrogen until the pressure in the autoclave is 3 atm, close the inflation valve; stir and react rapidly at a temperature of 40°C. During the reaction process, if the pressure drops, the hydrogen gas is supp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com