Antibacterial and antiviral medicinal composition and application thereof
An anti-virus and composition technology, applied in the field of medicine, can solve the problems of limited immune validity, low toxicity and side effects, high morbidity, mortality, etc., and achieve the effect of good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] An antibacterial and antiviral medicinal composition, the raw materials of which include 885g of patchouli, 1869g of herb, 1064g of fennel, 2174g of mugwort leaves, 46g of cloves, 532g of mint, and 10g of natural borneol.
[0081] Use above-mentioned medicinal composition to prepare medicinal volatile oil, preparation method is as follows:
[0082] Patchouli herb decoction pieces were crushed through a 20-mesh sieve, soaked overnight in 6 times the weight of water, extracted by steam distillation for 6 hours, and the volatile oil of patchouli was collected, with an extraction rate of 2.26%.
[0083] Atractylodes atractylodis crude powder was taken, soaked in 6 times the weight of water for 0.5h, extracted by steam distillation for 5h, and the volatile oil of atractylodis was collected, with an extraction rate of 1.07%.
[0084] Take the herb medicinal material, soak it in 6 times the weight of water for 2 hours, extract it by steam distillation for 3 hours, and collect ...
Embodiment 2
[0090] An antibacterial and antiviral medicinal composition, the raw materials of which include 663.5g of patchouli, 1402g of herb, 798g of fennel, 1630.5g of mugwort leaves, 32g of cloves, 106.5g of mint, and 2.5g of natural borneol.
[0091] Use above-mentioned medicinal composition to prepare the method for medicinal volatile oil, preparation method is as follows:
[0092] All raw materials are first crushed into coarse powder, after mixing, put in HA220-40-48 supercritical carbon dioxide extraction device, feed liquid carbon dioxide to extract volatile oil, extraction pressure is 28-30Mpa, extraction temperature is 55°C, separation temperature is 45°C, extraction time is 3h , to obtain 106.5 g of extract (total volatile oil), add natural borneol, and heat appropriately (not higher than 60° C.) to completely dissolve the natural borneol to obtain medicinal volatile oil.
Embodiment 1
[0094] Carry out drug efficacy research test to embodiment 1 medicinal volatile oil:
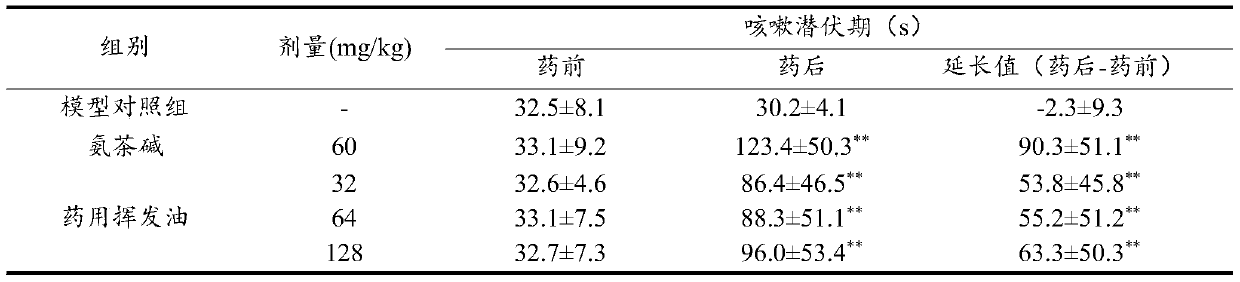
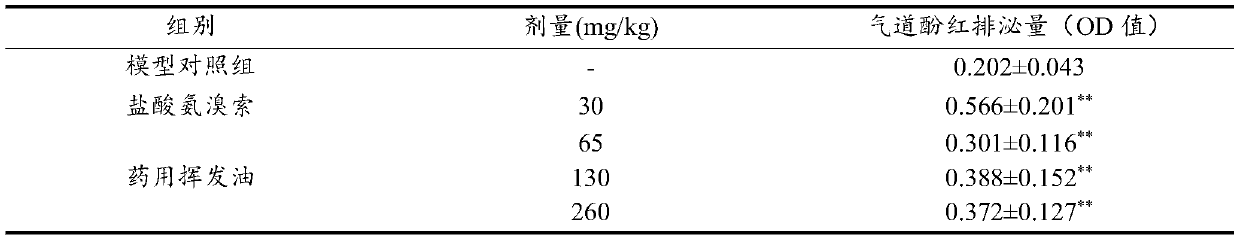
[0095] The doses of mice in the pharmacodynamic study were 65mg / kg, 130mg / kg and 260mg / kg, respectively.
[0096] The doses of guinea pigs in the drug efficacy study were 32mg / kg, 64mg / kg and 128mg / kg respectively.
[0097] The rat doses in the pharmacodynamic study were 37mg / kg, 74mg / kg and 148mg / kg
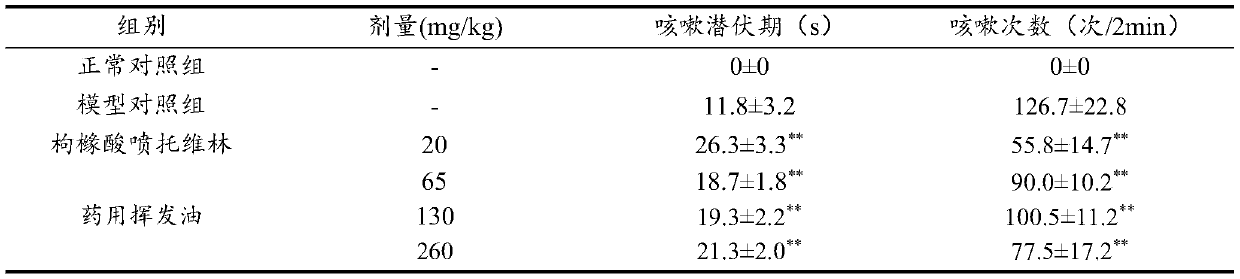
[0098] 1 Cough induced by concentrated ammonia water in mice (antitussive effect)
[0099] 1.1 Experimental method
[0100] ICR mice, half male and half female. According to weight stratification, they were randomly divided into 6 groups, 14 in each group, respectively: normal control group, model control group, pentoxyverine citrate 20mg / kg group, medicinal volatile oil low dose (65mg / kg) group, In the middle dose of medicinal volatile oil (130mg / kg) group and the high dose of medicinal volatile oil (260mg / kg) group, the mice were intragastrically administered at 20mL / kg (using soybean oi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com