Borane derivative and preparation method thereof and electroluminescent devices
A technology of electroluminescent devices and derivatives, applied in the direction of electric solid devices, luminescent materials, electrical components, etc., to achieve the effects of avoiding interface miscibility, avoiding interface erosion, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] Another embodiment of the present invention also provides a method for preparing borane derivatives, comprising the following steps:
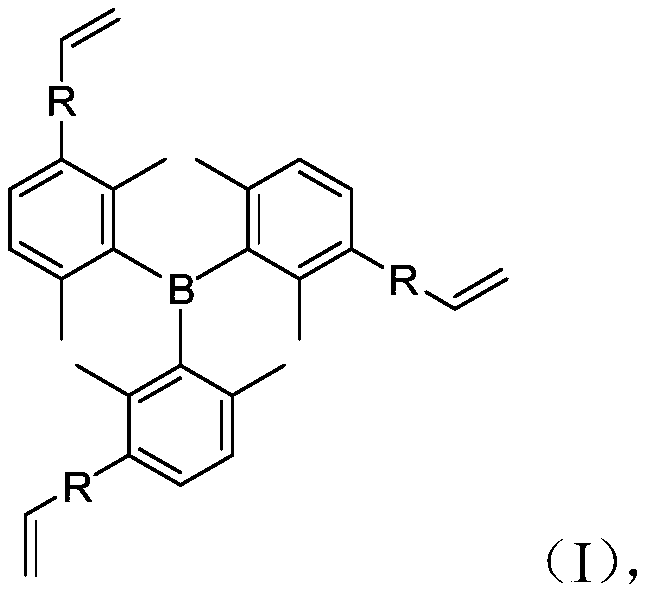
[0066] Substituting the compound of formula (a) with the compound of formula (b) to generate a borane derivative of formula (I);
[0067] The structural formulas of formula (a) compound, formula (b) compound and formula (I) borane derivative are as follows respectively:
[0068]
[0069] In formula (I), the structure of R is Ar 1 —Ar 2 , and Ar 2 Linked to the vinyl group in formula (I);
[0070] Among them, Ar 1 is a substituted or unsubstituted electron-deficient aromatic heterocyclic group, Ar 2 is a substituted or unsubstituted electron-rich aromatic ring group.
[0071] Specifically, using the compound of formula (a) and the boronic acid vinyl derivative of formula (b) as raw materials, tris(dibenzylideneacetone) dipalladium as a catalyst, and tricyclohexylphosphine as a ligand, in an inert protective atmosphere , under or...
Embodiment 1
[0098] The synthesis of embodiment 1 borane derivatives
[0099] The first step: Synthesis of bromobenzene derivatives (1)
[0100]
[0101] First vacuumize the 50mL two-necked bottle and change the nitrogen gas operation, repeat three times, so that the pressure tube is in a nitrogen atmosphere, and then add 9mmol and 25mL of anhydrous tetrahydrofuran (THF), dissolved, placed at a low temperature of -78°C for 15min, then added 9mmol of n-butyl lithium (n-BuLi) dropwise, and carried out a constant temperature reaction at -78°C for 3h, the reaction ended After that, add 3mmol boron fluoride diethyl ether (BF 3 O(C 2 h 5 ) 2 ), and kept at -78°C for 12 hours. After the reaction was completed, the product was separated and purified with a silica gel chromatography column, using n-hexane / dichloromethane as eluent, and the product bromobenzene derivative (1) was collected by rotary evaporation and solvent removal, and finally vacuum-dried at room temperature for 12 hours a...
Embodiment 2
[0143] Embodiment 2 A kind of electroluminescent device
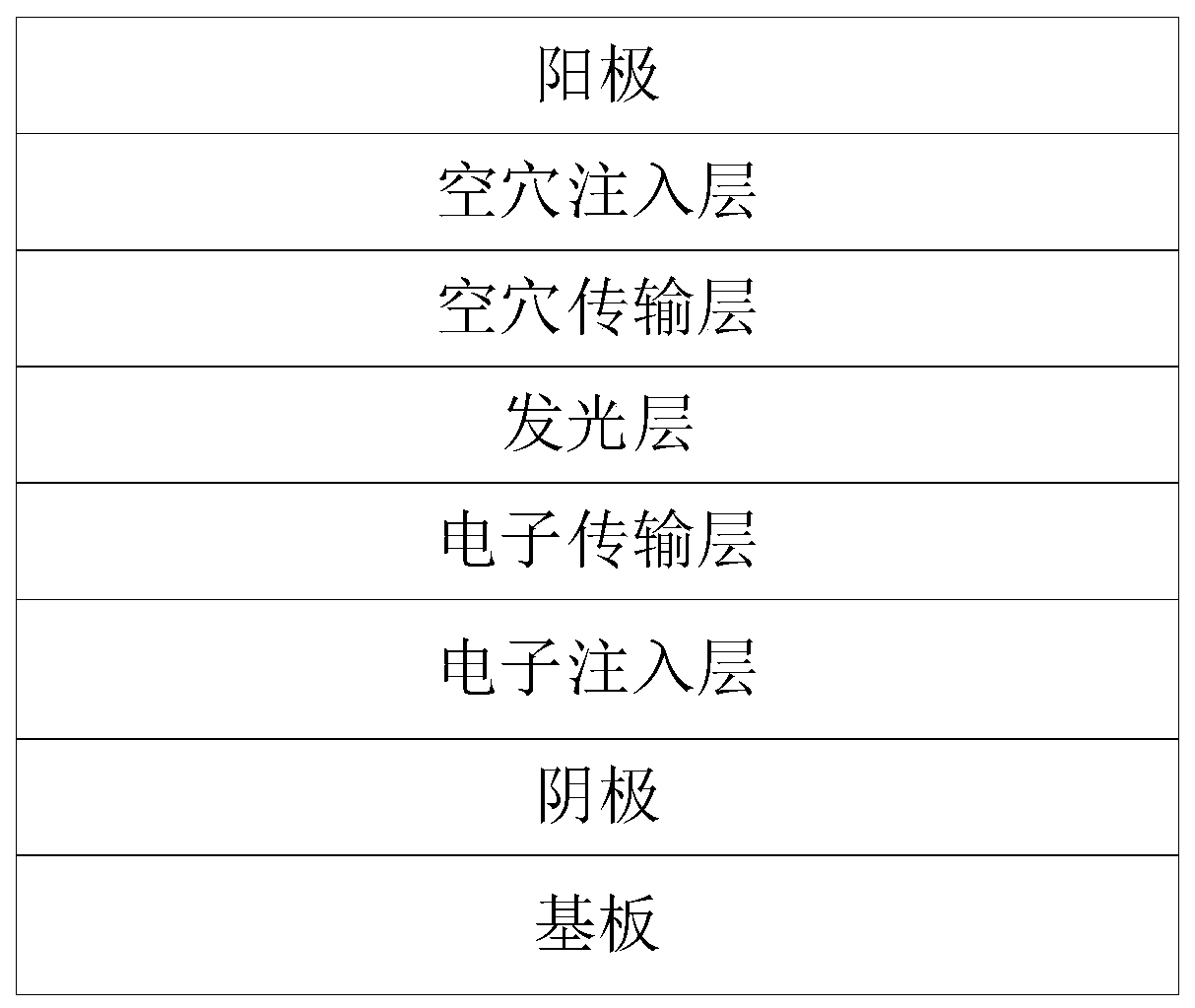
[0144] The device structure of this embodiment is: ITO / ZnO(35nm) / crosslink-M1(20nm) / mCP:Ir(ppy) 2 acac, 7wt% (30nm) / TAPC(30nm) / NPB(10nm) / HAT-CN(10nm) / Al(120nm).
[0145] Among them, the cathode is ITO; ZnO is used as the material of the electron injection layer; the cross-linked borane derivative M1 is used as the material of the electron transport layer; mCP and Ir(ppy) 2 acac is used as the light-emitting layer material, among which, mCP is used as the host material, and Ir(ppy) 2 acac as the guest material, Ir(ppy) 2 The doping concentration of acac is 7wt%; TAPC is used as the material of one layer in the hole transport layer, NPB is used as the material of the other layer in the hole transport layer; HAT-CN is used as the material of the hole injection layer; Al is used as the anode.
[0146]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com