Method for efficiently expressing ORF2 (Open Reading Frame 2) gene of goose astrovirus soluble capsid precursor, and application of method
An astrovirus and capsid protein technology, which is applied in the field of the ORF2 gene that efficiently expresses the soluble capsid protein of goose astrovirus, can solve the problems of lack of preventive and therapeutic products, difficulty in controlling the source of infection, and prolonging the emptying time, etc. To achieve the effect of cost reduction, high protein activity and good protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Expression of Goose Astrovirus ORF2 Gene in Escherichia coli Prokaryotic Expression Vector
[0030] 1. Synthesis of ORF2 gene
[0031] Referring to the ORF2 gene sequence of the Goose Astrovirus strain, the sequence was optimized by comprehensive factors, such as the codon bias of Escherichia coli. Artificially synthesized ORF2 gene, as shown in SEQ ID NO.1, was connected into the vector to obtain pUC57-ORF2.
[0032] 2. Design and PCR amplification of cloned genes
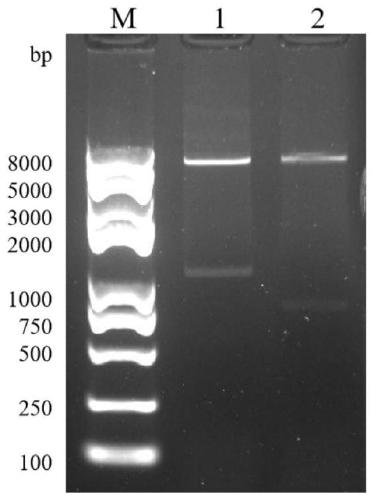
[0033] The ORF2 gene was divided into two segments of capsid protein 39nt-406nt and spike protein 394nt-665nt according to the different encoded proteins for vector construction and expression respectively.
[0034] Primers were designed according to the nucleotide sequence for gene amplification. The primer sequence was Seg1-F CGCGGATCCCCAGAAACTGCCGATGAAAGCTGAAC; Seg1-R AAAAGGAAAAGCGGCCGCGCCCTGACCAGTGGTGTTAACGTTC was used to amplify the capsid protein of 368 amino acids; Seg2-F CGCGGATCCATGCAGGTGACCCCGAG...
Embodiment 2
[0051] Construction of Astrovirus recombinant Escherichia coli expressing cap protein and spike protein
[0052] 1. Construction and expression of recombinant strains
[0053] (1) Transform the correctly identified recombinant plasmids pET28a-cap and pET28a-spike into Escherichia coli BL21(DE3)pLysS, respectively, to construct recombinant expression strains BL21(DE3)pLysS(pET28a-cap) and BL21(DE3)pLysS(pET28a- spike).
[0054] (2) After activating BL21(DE3)pLysS(pET28a-cap) and BL21(DE3)pLysS(pET28a-spike) respectively, they were inoculated into liquid TB (Kan, 50μg / ml) medium at a ratio of 1:100, Place in a shaker at 37°C for about 3 hours, add IPTG to a final concentration of 0.5mM, centrifuge the bacterial solution after overnight induction, remove the TB supernatant, and resuspend the remaining bacterial cells in PBS for ultrasonic disruption.
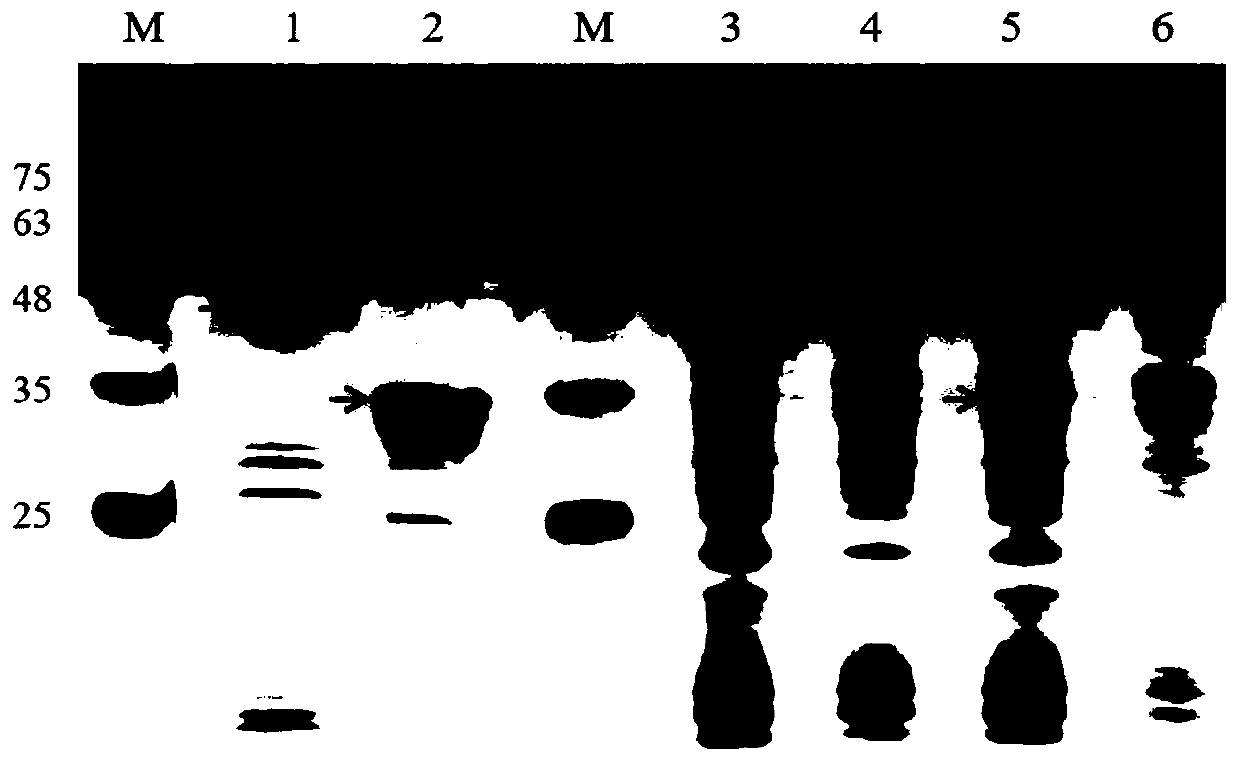
[0055] (3) The supernatant and the precipitate were separated by centrifugation of the sonicated bacteria, and the solubility w...
Embodiment 3
[0063] Affinity chromatography purification of cap and spike proteins
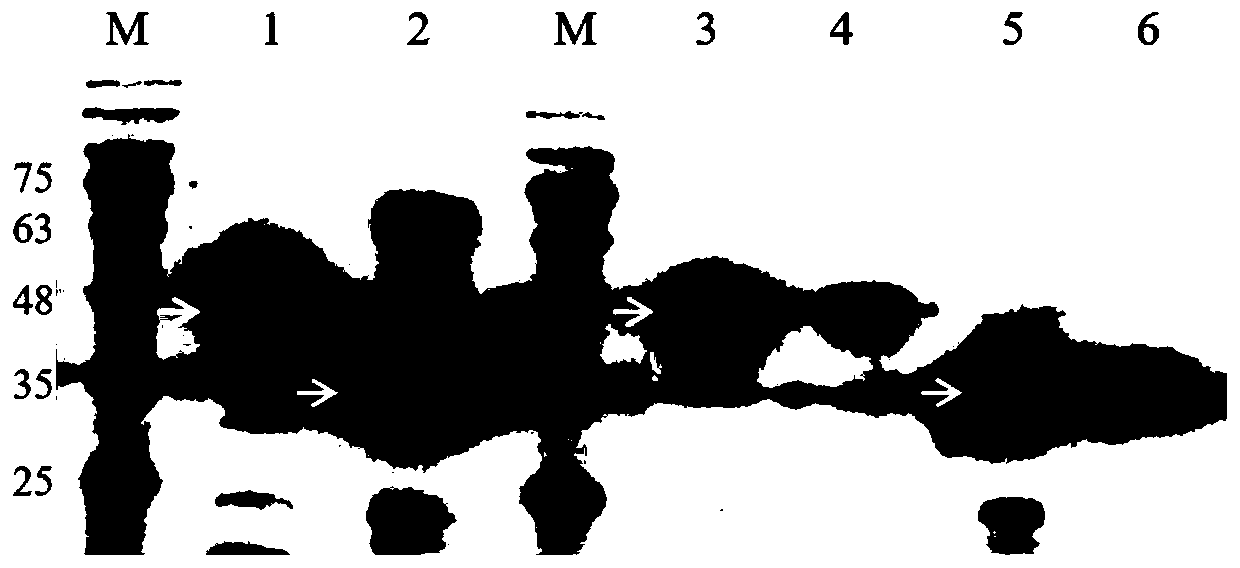
[0064] The broken supernatant of Escherichia coli periplasm was purified by Ni-NTA chromatographic column. 2 O slowly flows through the nickel column, 20% ethanol solution flows out, and the column is washed repeatedly; (2) Equilibrium nickel column: 20mL Binding Buffer balance column; (3) Sample loading: add 10mL cap protein or spike protein to the nickel column , repeated loading 3 times; (4) washing protein: 50mL Washing Buffer continuously washes miscellaneous protein; (5) elution protein: 10mL Elution Buffer elutes the target protein; the purified sample is identified by SDS-PAGE and Western Blot, cap protein The purity of spike protein can reach more than 90% after affinity chromatography purification. The results of Western blot identification are as follows: image 3 Lane 1 and Lane 2 show: the purified cap and spike proteins were subjected to Western blot with his antibody, which showed that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com