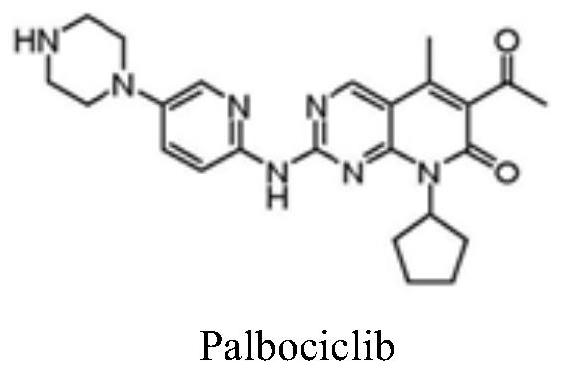
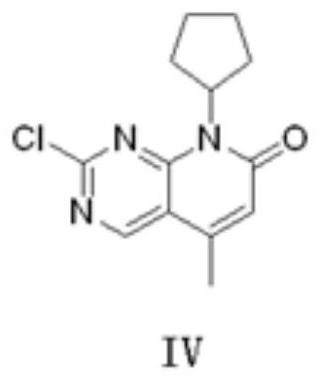
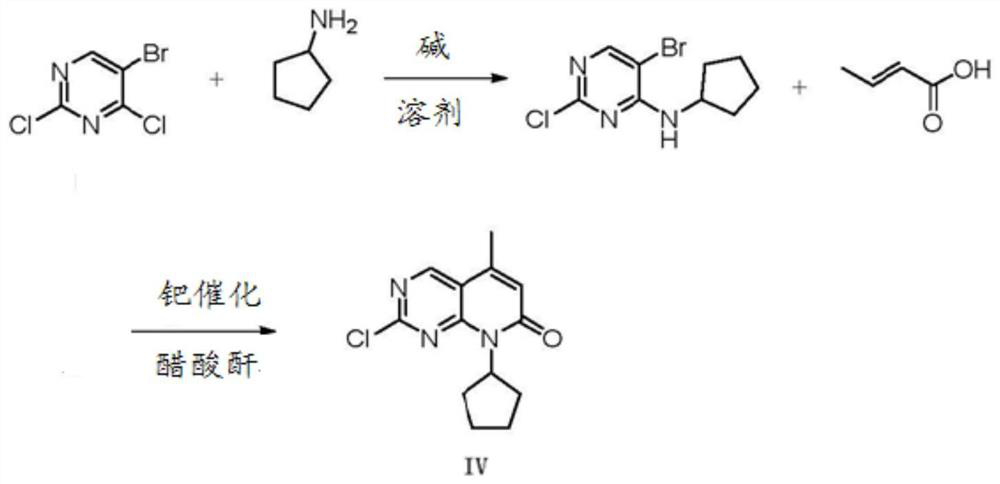
A kind of preparation method of palbociclib intermediate
A technology of intermediates and compounds, applied in the field of drug synthesis, can solve problems such as unfavorable intermediates industrial production, difficult control of production costs, complex reaction products, etc., to avoid the use of heavy metal palladium catalysts, large implementation value, and reaction yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Preparation of compound II:
[0031] The ratio of compound I: 2,4-dichloropyrimidine, cyclopentylamine and triethylamine is =1:1:1.2
[0032] Add (1490g, 10.0mol) 2,4-dichloropyrimidine and 3L ethanol to a 10L four-neck flask, control the temperature in an ice-water bath to not exceed 25°C, add (851g, 9.99mol) cyclopentylamine and (1215g, 12.0mol) dropwise ) a mixed solution of triethylamine, after the dropwise addition was completed, stir at room temperature for 1 h. After the reaction was detected by HPLC, 3 L of water was added, stirred at room temperature for 1 hour to precipitate a white solid, filtered, washed with water, and dried to obtain compound II: 1680 g of 2-chloro-N-cyclopentylpyrimidin-4-amine, yield: 85.0%, MS: m / z 199 (M+H) +
[0033] The hydrogen spectrum characterization of compound II: 1 H NMR (400MHz, CDCI 3 , δppm): 1.46-1.56 (m, 4H), 1.58-1.83 (m, 4H), 2.64 (m, 1H), 6.14 (d, 1H), 7.59 (s, 1H), 7.73 (d, 1H).
[0034] 2) Preparation of Com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com