Medicine for treating spinal cord injury, medicine kit and method
A spinal cord injury and kit technology, applied in the field of biomedical technology, can solve problems such as limited functional recovery, and achieve the effects of promoting functional recovery, improving regenerative ability, and enhancing regenerative ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Construction of pinch injury model with overexpression of OPN, IGF1 and T10 in neurons
[0032] Healthy adult female SD rats, SPF grade, weighing 220-250 g. After the rats were weighed, compound anesthetic (0.3ml / 100g) was injected intraperitoneally. After the rats were completely anesthetized, their backs were shaved and disinfected with povidone iodine. The skin and muscle were incised in sequence, and the T8 segment of the spinal cord was located through the bony landmarks. Part of the lamina was removed to expose the spinal cord. The upper and lower vertebrae were fixed through rat spinal cord adapters. OPN and AAV-IGF1 were injected into the spinal cord parenchyma through a digital three-dimensional injection device. A total of 8 injection points were evenly distributed along both sides of the T8 segment. Each injection point was injected at 3 depths, respectively 1.5mm, 1.0mm, and 0.5mm (Virus titer: 1.15×10 13 gc / ml, injection volume: 200nl / point,...
Embodiment 2
[0033] Example 2. Preparation and transplantation of embryonic spinal cord neural stem cells (NSCs)
[0034] 14 days pregnant green fluorescent rats were executed by decapitation after deep anesthesia. After the ventral side was disinfected with 75% alcohol, the skin and muscles were cut open in turn to fully expose them. The fetuses were taken out and placed in a petri dish containing pre-cooled PBS. Identify GFP fetal mice with fluorescent detection lamps; cut off the head and tail of the identified fetal mice, transfer them to a clean petri dish, place the ventral side down, stretch the limbs, find the dorsal midline under a dissecting microscope, and microscissor Gently cut the transparent skin layer from the head to the tail to expose the spinal cord, completely separate the spinal cord from the surrounding tissues, cut off, place in a clean petri dish, and wash 2-3 times in pre-cooled saline; Under the microscope, the dura mater, blood vessels, and possibly attached DR...
Embodiment 3
[0036] Example 3, small molecule compound CLP290 intervention
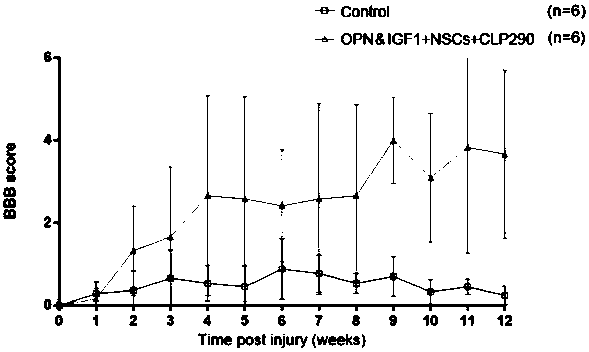
[0037] From the 4th week after operation, 0.2ml small molecular compound CLP290 (concentration: 7mg / ml) was injected intraperitoneally every day until the end of the experiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com