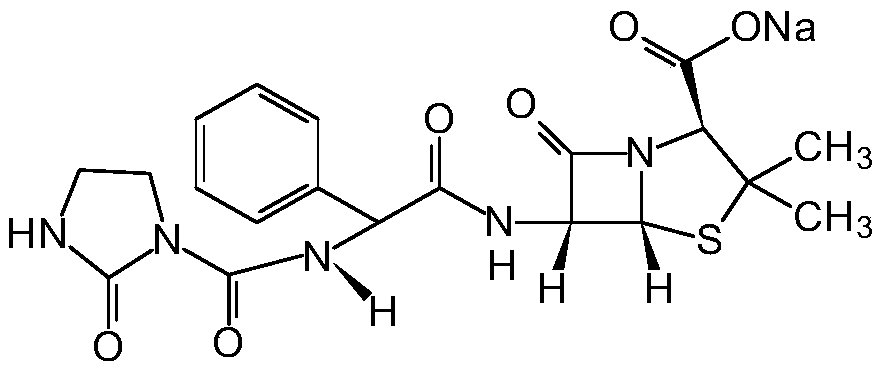
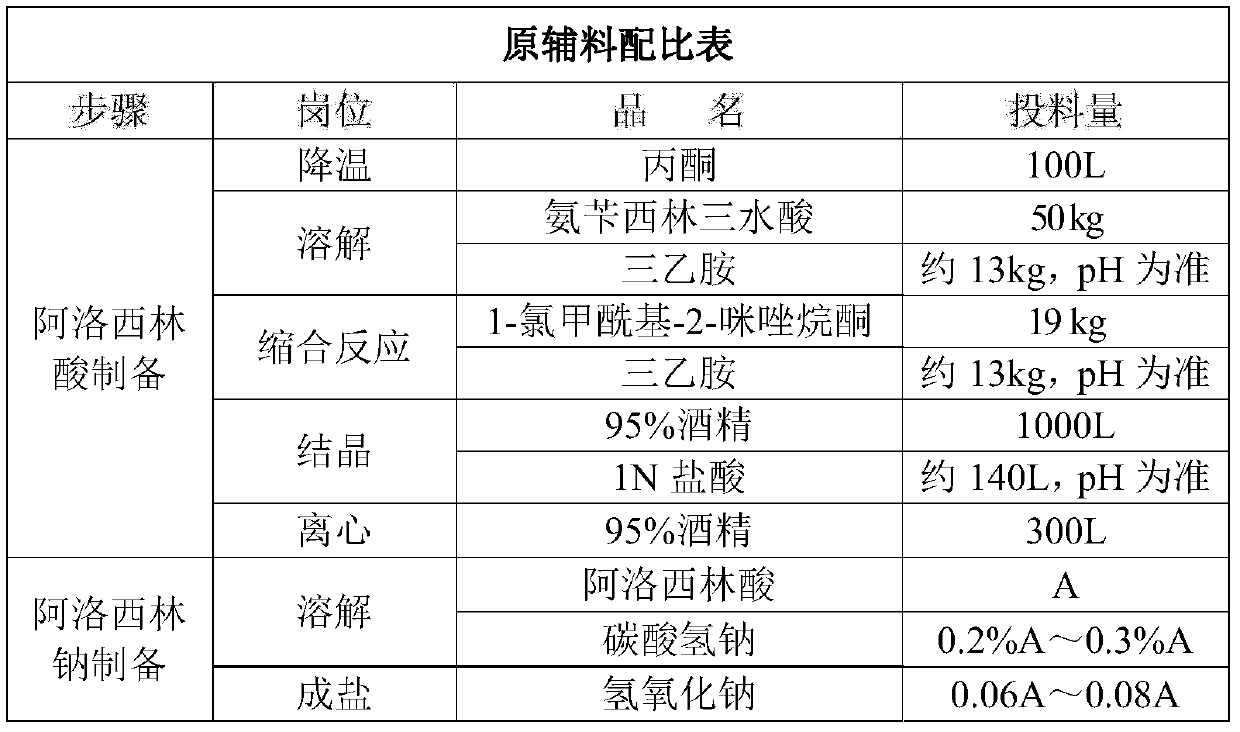
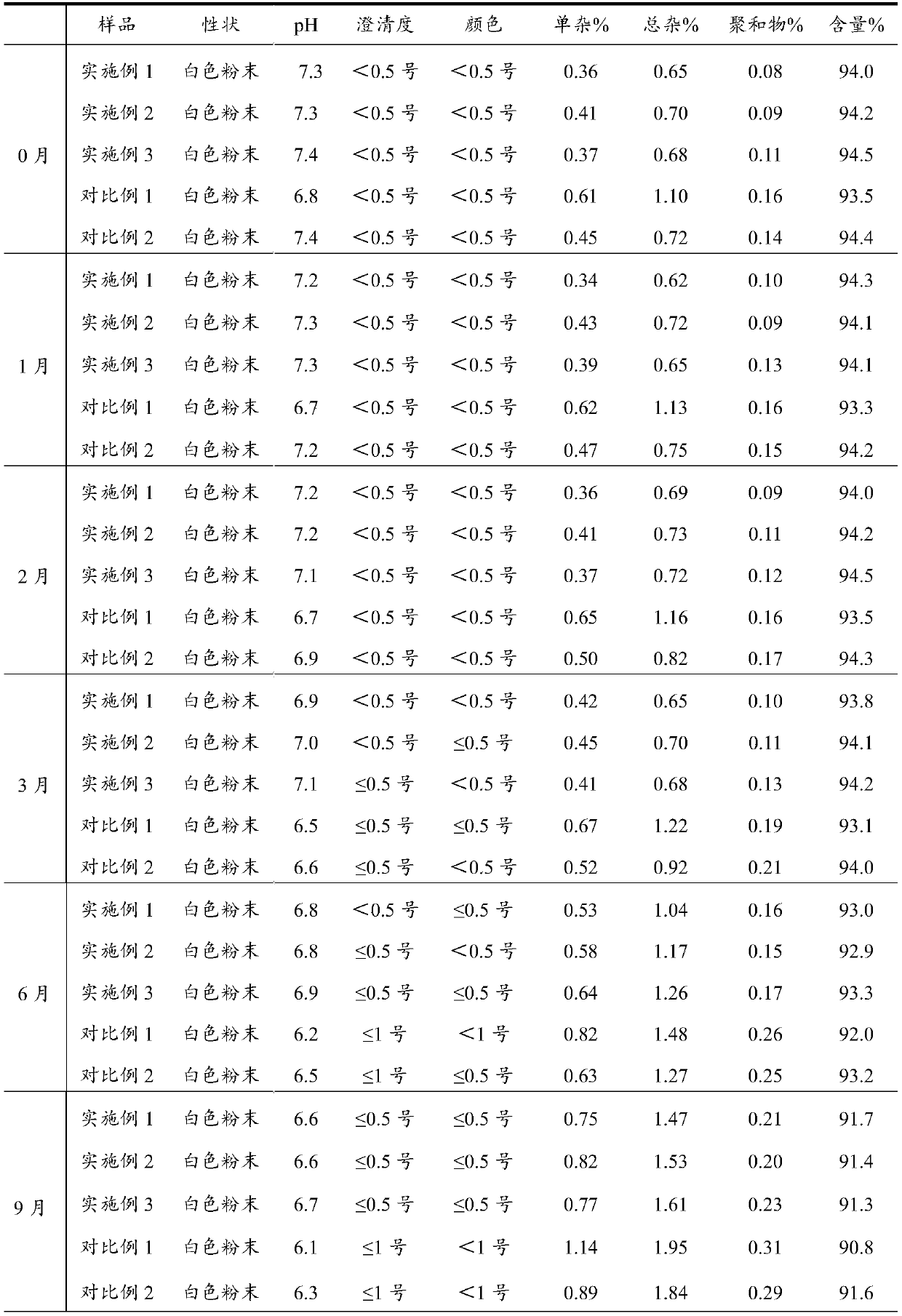
Preparation process of azlocillin sodium
A technology of azlocillin sodium and preparation process, applied in the direction of organic chemistry and the like, can solve the problems of poor storage stability, high impurity content, poor use safety, etc., and achieve the effects of improving stability, increasing yield and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Add 500L of purified water and 100L of acetone into the glass-lined reactor, stir and cool down to 0-5°C;
[0049] (2) Add 50kg of ampicillin trihydrate, add about 13kg of triethylamine at 0-5°C, stir and dissolve for 30min, and the pH is 8.0-9.0;
[0050](3) After the reaction solution was dissolved, quickly add 19 kg of 1-chloroformyl-2-imidazolidinone into the reaction kettle. After the addition is complete, stir at 0-5°C for 1 hour, add about 13 kg of triethylamine, continue to stir and dissolve for 30 minutes, and the pH is 8.0-9.0;
[0051] (4) After dissolving, filter the reaction solution into the crystallization tank through a 0.45 μm filter element;
[0052] (5) Add 1000L of 95% ethanol to the crystallization tank, stir and cool down to 10-15°C;
[0053] (6) Slowly add 1N hydrochloric acid dropwise at 10-15°C, adjust pH=2.5-3.0, and continue stirring for 0.5 hours;
[0054] (7) Put the material into a centrifuge for centrifugation. After the centrifuga...
Embodiment 2
[0068] (1) Add 500L of purified water and 100L of acetone into the glass-lined reactor, stir and cool down to 0-5°C;
[0069] (2) Add 50kg of ampicillin trihydrate, add about 13kg of triethylamine at 0-5°C, stir and dissolve for 30min, and the pH is 8.0-9.0;
[0070] (3) After the reaction solution was dissolved, quickly add 19 kg of 1-chloroformyl-2-imidazolidinone into the reaction kettle. After the addition is complete, stir at 0-5°C for 1 hour, add about 13 kg of triethylamine, continue to stir and dissolve for 30 minutes, and the pH is 8.0-9.0;
[0071] (4) After dissolving, filter the reaction solution into the crystallization tank through a 0.45 μm filter element;
[0072] (5) Add 1000L of 95% ethanol to the crystallization tank, stir and cool down to 10-15°C;
[0073] (6) Slowly add 1N hydrochloric acid dropwise at 10-15°C, adjust pH=2.5-3.0, and continue stirring for 0.5 hours;
[0074] (7) The material is released to the centrifuge for centrifugation. After the c...
Embodiment 3
[0088] (1) Add 500L of purified water and 100L of acetone into the glass-lined reactor, stir and cool down to 0-5°C;
[0089] (2) Add 50kg of ampicillin trihydrate, add about 13kg of triethylamine at 0-5°C, stir and dissolve for 30min, and the pH is 8.0-9.0;
[0090] (3) After the reaction solution was dissolved, quickly add 19 kg of 1-chloroformyl-2-imidazolidinone into the reaction kettle. After the addition is complete, stir at 0-5°C for 1 hour, add about 13 kg of triethylamine, continue to stir and dissolve for 30 minutes, and the pH is 8.0-9.0;
[0091] (4) After dissolving, filter the reaction solution into the crystallization tank through a 0.45 μm filter element;
[0092] (5) Add 1000L of 95% ethanol to the crystallization tank, stir and cool down to 10-15°C;
[0093] (6) Slowly add 1N hydrochloric acid dropwise at 10-15°C, adjust pH=2.5-3.0, and continue stirring for 0.5 hours;
[0094] (7) The material is released to the centrifuge for centrifugation. After the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com