Antibacterial amidine oligomer with drug resistance, and preparation method and application thereof
A production method and oligomerization-like technology, which are applied in the field of medicine and chemical industry, can solve the problems of large side effects, high cytotoxicity and hemolytic activity of antibacterial polymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] After testing, the present invention has developed 8 antibacterial amidine oligomers with broad-spectrum antibacterial properties, and its chemical formula is as follows:
[0042]
[0043] where 5≤n≤8;
[0044] Wherein the molecular formula of R is as shown in formula (I), formula (II), formula (III), formula (IV), formula (V), formula (VI), formula (VII) or formula (VIII);
[0045]
[0046]
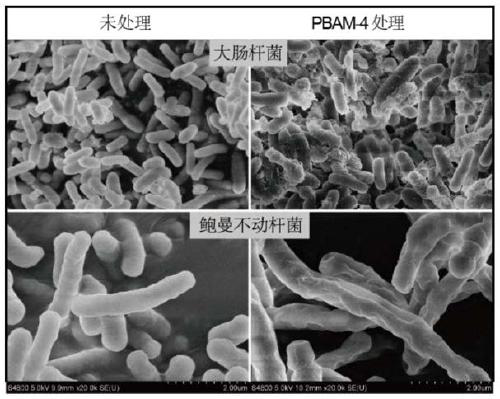
[0047] For convenience of record, the molecular formula of R is the compound when formula (I), formula (II), formula (III), formula (IV), formula (V), formula (VI), formula (VII) and formula (VIII) They are respectively numbered as PBAM-1, PBAM-2, PBAM-3, PBAM-4, PBAM-5, PBAM-6, PBAM-7, PBAM-8. The antibacterial properties of the 8 compounds are shown in Table 1 and Table 2:
[0048] Antibacterial properties of 8 kinds of compounds in table 1
[0049]
[0050]
[0051] Antibacterial properties of 8 compounds in table 2
[0052]
[0053]
[0054]in, a Indic...
Embodiment 2
[0070] In order to expand the types of antibacterial amidine oligomers, different H 2 N-R-NH 2 As a mixed raw material to prepare antibacterial amidine oligomers, it is found that the obtained mixed product also has obvious antibacterial properties, as shown in Table 3:
[0071] Table 3 Mixed raw materials prepare the antibacterial properties of antibacterial amidine oligomers
[0072]
[0073]
[0074] Table 3 shows that the R group of the antibacterial amidine oligomer is a mixed group and also has antibacterial properties.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com