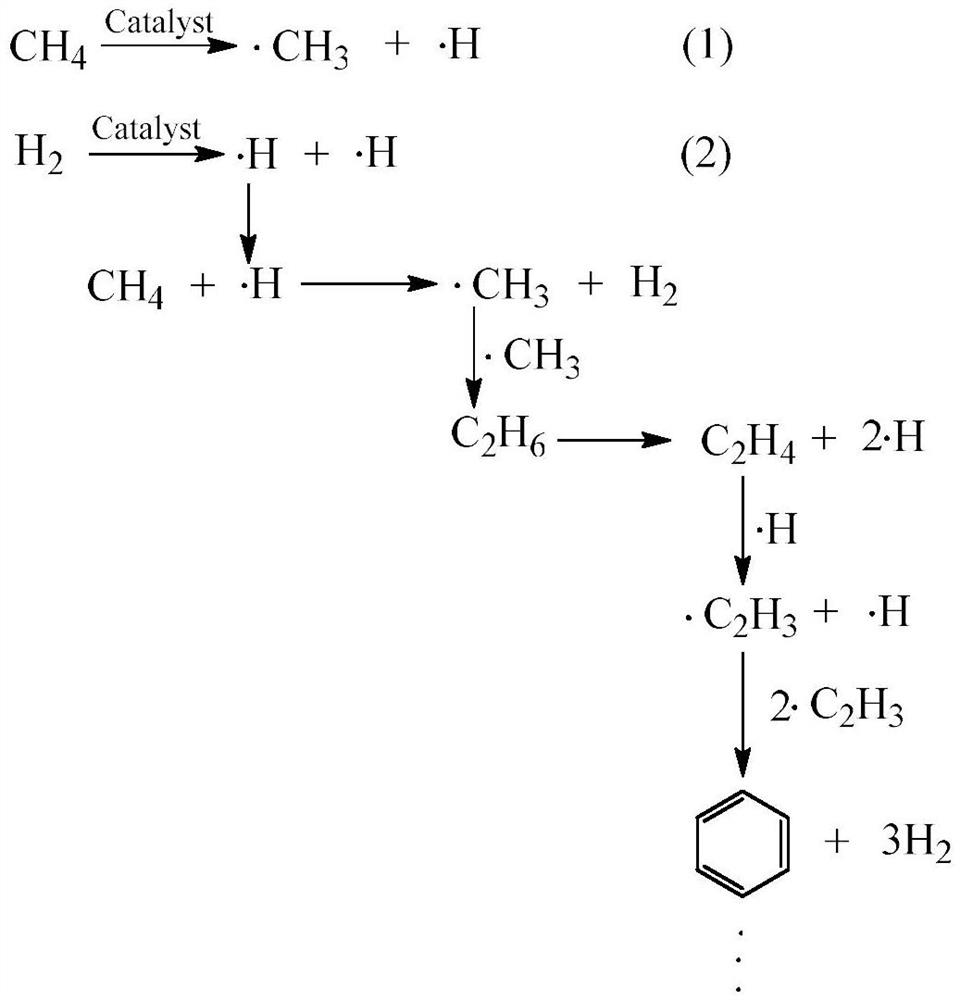
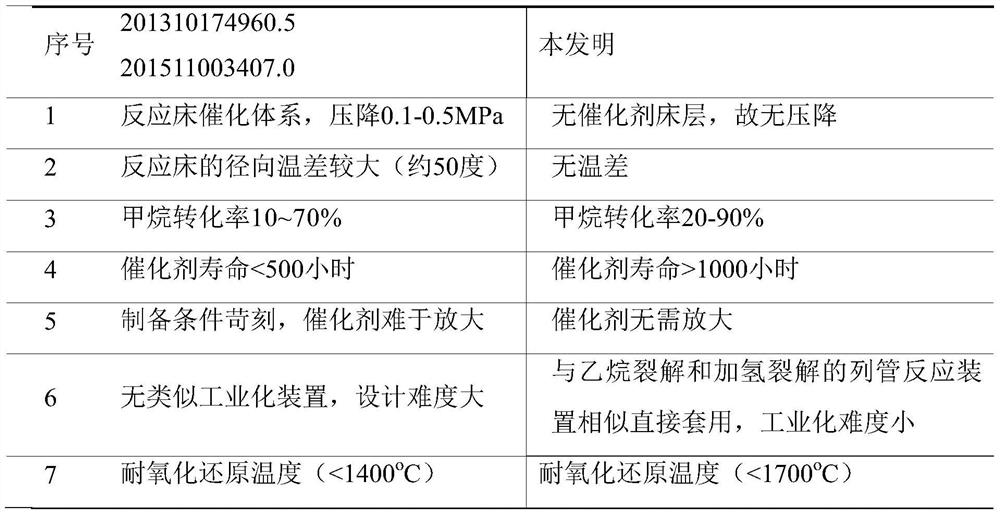
A method for catalytic conversion of methane to olefins, aromatics and hydrogen under the condition of hydrogen
A hydrogen and methane technology, applied in the field of catalytic conversion of methane to olefins, aromatics and hydrogen under hydrogen conditions, can solve problems such as difficult scale application, difficult scale-up, and low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] 1. Preparation of catalytic reactor (thin layer thickness and active component content need to be indicated)
[0062] The preparation method of the lattice doped catalyst includes chemical vapor deposition (MCVD) coating solid-phase doping technology or solid-liquid phase sol-gel combined with high-temperature melting coating technology. Membrane catalysts are marked as:
Embodiment 1
[0065] Modified Chemical Vapor Deposition (MCVD)
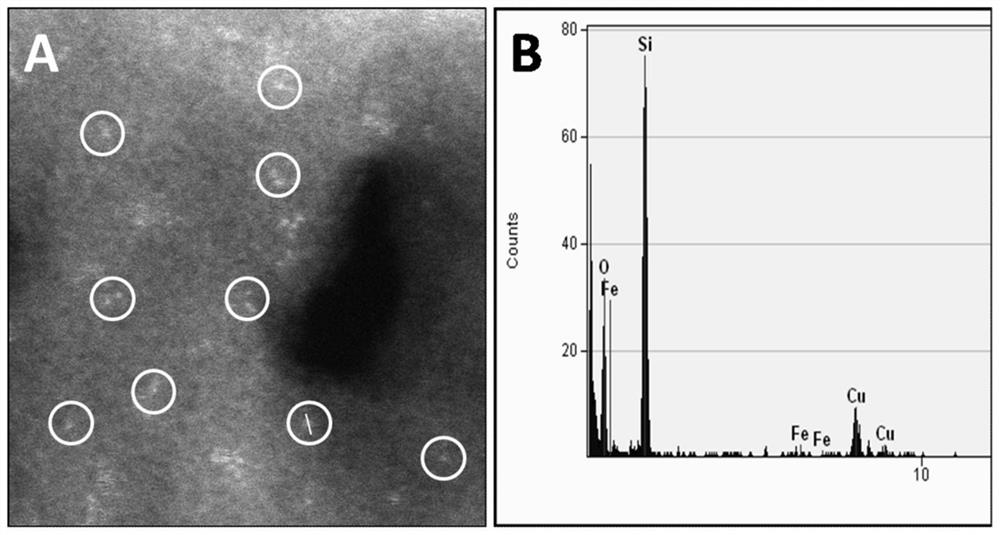
[0066] Use 30mL / min of high-purity oxygen to SiCl 4 Liquid and FeCl under saturated vapor pressure at 350°C using 200mL / min high-purity helium 3 The gas is brought into the high-temperature MCVD device, and the inner wall of the quartz tube (wall thickness 1.5mm) with an outer diameter of 20 mm and a length of 100 mm is heated at 1600 ° C by SiCl 4 and FeCl 3 After 10 min of oxide deposition, Fe-doped SiO was obtained 2 The powder material is then melted for 40 minutes at a temperature of 1980°C under a 2bar high-purity helium atmosphere to form a thin layer of dopant with a thickness of 100nm on the inner wall of the reactor, and then naturally cooled to obtain a 20mm in diameter and 100mm in length Catalytic quartz reactor, wherein Fe doping amount is 0.05wt.%.
Embodiment 2
[0068] Modified Chemical Vapor Deposition (MCVD)
[0069] Use 30mL / min of high-purity oxygen to SiCl 4 Liquid and FeCl under saturated vapor pressure at 350°C using 650mL / min high-purity helium 3 The gas is brought into the high-temperature MCVD device, and the inner wall of the quartz tube (wall thickness 1.5mm) with an outer diameter of 20 mm and a length of 100 mm is heated at 1600 ° C by SiCl 4 and FeCl 3 After 10 min of oxide deposition, Fe-doped SiO was obtained 2 The powder material is then melted for 40 minutes at a temperature of 1980°C under a 2bar high-purity helium atmosphere to form a thin layer of dopant with a thickness of 100nm on the inner wall of the reactor, and then naturally cooled to obtain a 20mm in diameter and 100mm in length Catalytic quartz reactor, wherein Fe doping amount is 0.1wt.%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com