A recombinant novel coronavirus vaccine based on human replication-defective adenovirus
A coronavirus, recombinant adenovirus technology, applied in the direction of viruses/phages, viruses, viral peptides, etc., to achieve the effects of good immunogenicity, fast and simple preparation method, and good immune protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
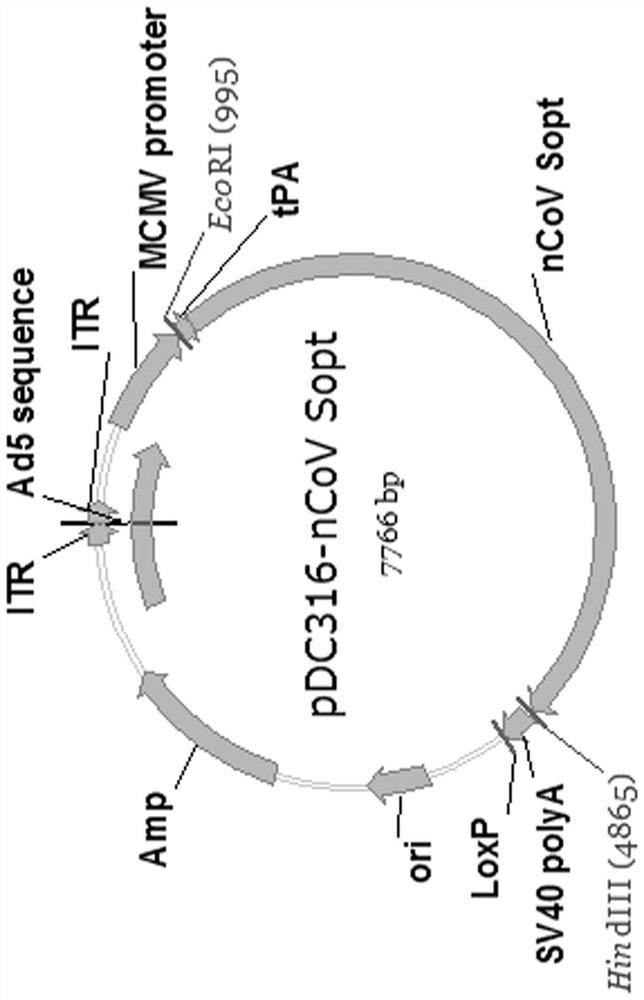
[0043] Example 1. Preparation of recombinant novel coronavirus vaccine with human replication defective adenovirus as carrier
[0044] 1. S protein gene optimization and synthesis
[0045] The target antigen of the recombinant novel coronavirus vaccine is the S protein of the novel coronavirus strain (Genebank number: NC_045512.2). By optimizing the S protein gene, the expression level of the S protein is increased, thereby improving the immunogenicity of the vaccine.
[0046] First, use the Upgene software (Gao, W.Rzewski, A.Sun, H.Robbins, P.D. & Gambotto, A. UpGene: Application of a web-based DNA codon optimization algorithm. Biotechnol Prog, 2004.20 (2): p.443-8 .) Perform codon optimization to change most of the rare codons in the S protein gene to high frequency codons. Secondly, considering that software optimization may mechanically change codons to the most frequently used codons, protein translation efficiency may not be significantly improved due to the influence ...
Embodiment 2
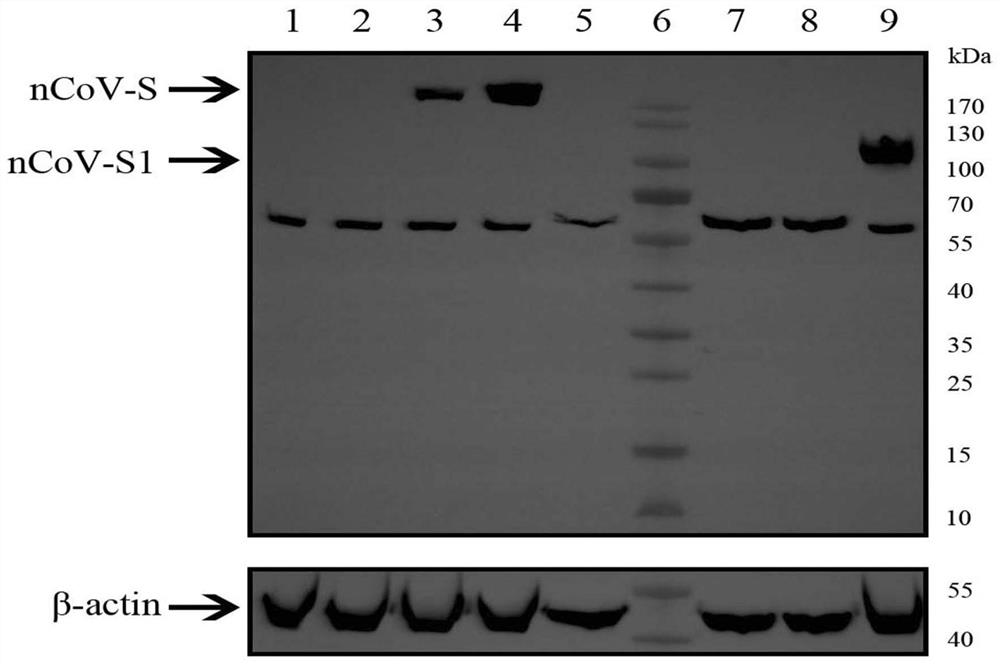
[0107] Example 2. Immunological evaluation of different constructed recombinant adenoviruses on mouse models
[0108] 1. Vaccine humoral immune response detection
[0109] 100 SPF grade female BALB / c mice (6-8 weeks old) were randomly divided into 10 groups, 10 mice in each group. The mice were immunized with Ad5-nCoV according to the grouping conditions shown in Table 1. The way of intramuscular injection is to inject 100 μL into the inner side of the hind thigh, and the way of nasal drop immunization is to anesthetize the mice with isoflurane and instill 20 μL through the nasal cavity. The grouping situation is shown in Table 1.
[0110] Table 1. Grouping of mice for vaccine humoral immune response detection
[0111]
[0112]
[0113] Blood was collected from the mice at a specific time point after immunization, the serum was separated, and the IgG antibody titer against the new coronavirus S protein in the serum was detected by ELISA. Test results such as Figure...
Embodiment 3
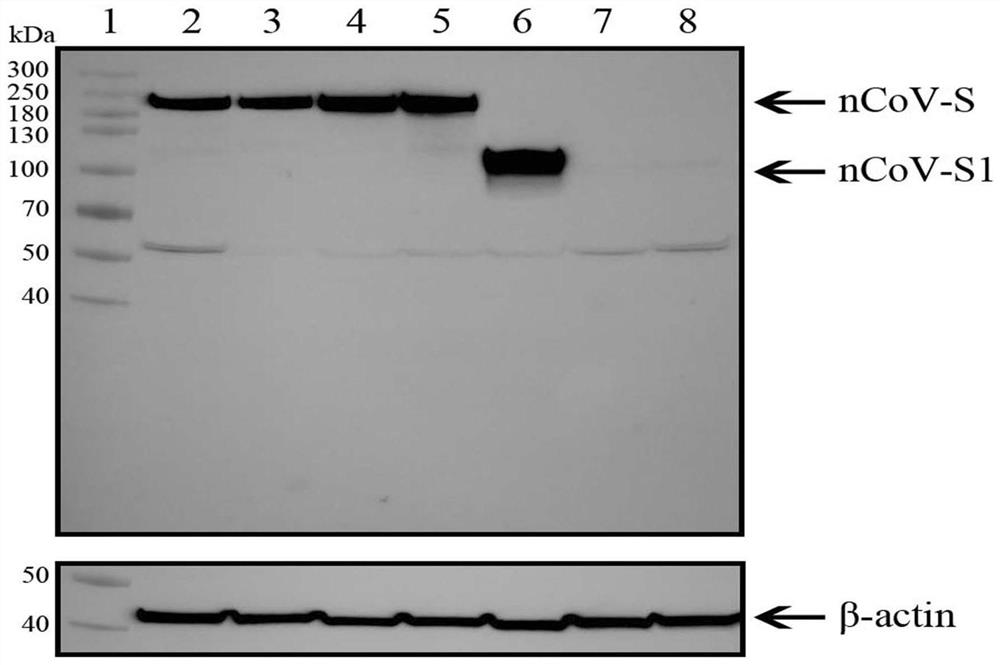
[0127] Example 3. Immunological evaluation of Ad5-nCoV on guinea pig model
[0128] Fifty-six guinea pigs of SPF grade, weighing 200 to 250 grams, were randomly divided into 4 groups, 14 in each group, half male and half male. Guinea pigs were immunized with Ad5-nCoV according to the grouping conditions shown in Table 3. The way of immunization was intramuscular injection of 200 μL in the rear thigh.
[0129] Table 3. Grouping of Ad5-nCoV guinea pig immunogenicity detection
[0130]
[0131] Blood was collected from the guinea pigs at a specific time point after immunization, the serum was separated, and the IgG antibody titer against the novel coronavirus S protein in the serum was detected by ELISA. Test results such as Figure 14 Shown (ns, P≥0.05; *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001). The results showed that 14 days after Ad5-nCoV immunization in guinea pigs, high levels of serum IgG antibody titers were detected. There was no significant difference...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com