Empagliflozin tablet and preparation process thereof
A technology for empagliflozin tablets and empagliflozin, which is applied in the field of empagliflozin tablets and their preparation, can solve the problems of explosion risk, reduced production efficiency, easy agglomeration and agglomeration, and achieves increased drug stability, increased Absorption and bioavailability, the effect of improving drug dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0087]
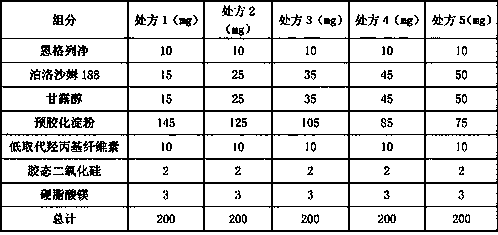
[0088] (1) Weigh the prescription amount of poloxamer 188 and mannitol, mix evenly, and heat to melt at 170°C;
[0089] (2) Add Empagliflozin under the condition of slow stirring at 200-350r / min, and stir vigorously at 10000-20000r / min until it melts;
[0090] (3) The completely melted mixture obtained in (2) was frozen at -20°C for 5 hours, vacuum-dried at 40°C for 5 hours, taken out, crushed, and passed through an 80-mesh sieve to obtain a solid composition;
[0091] (4) Mix the obtained solid composition with the prescribed amount of pregelatinized starch, croscarmellose sodium, colloidal silicon dioxide and magnesium stearate, and further make tablets.
[0092] Dissolution method: according to the "Chinese Pharmacopoeia" 2015 edition four dissolution and release test method (general rule 0931 second method)
[0093] Temperature: 37℃±0.5℃
[0094] Dissolution medium: 0.1mol / L hydrochloric acid solution
[0095] Medium volume: 900ml
[0096] Speed: 50 rpm
...
experiment example 2
[0103]
experiment example 3
[0114]
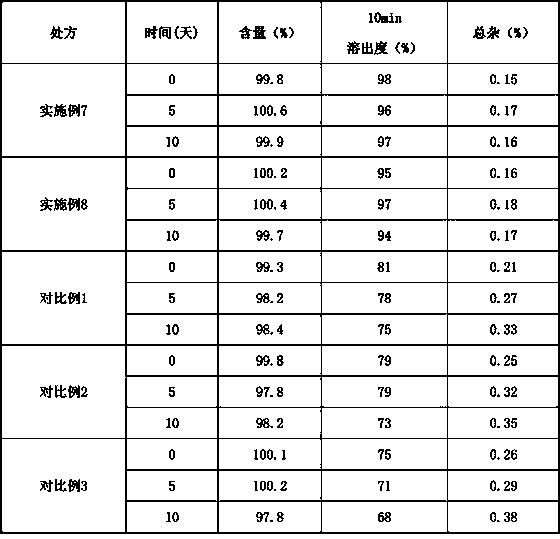
[0115] According to the preparation method, the superior prescription was prepared again, and the relevant physical properties were investigated.
[0116] Formability data
[0117]
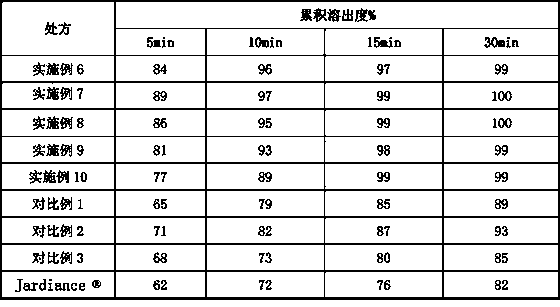
[0118] Dissolution Data
[0119] Dissolution in 0.1mol / L hydrochloric acid: According to the dissolution and release test method of the fourth part of "Chinese Pharmacopoeia" 2015 edition (general rule 0931 second method)
[0120] Temperature: 37℃±0.5℃
[0121] Dissolution medium: 0.1mol / L hydrochloric acid solution
[0122] Medium volume: 900ml
[0123] Speed: 50 rpm
[0124] Sampling: Take the solution after 5 minutes, 10 minutes, 15 minutes, and 30 minutes, filter it with a 0.45 μm microporous membrane, and replenish the corresponding dissolution medium at the same temperature in the operating container in time.
[0125] Detection method: HPLC method.
[0126]
[0127] Dissolution in pH 6.8 phosphate buffer solution: according to the dissolution and release test method o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Medium volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com