A kind of hydrogen peroxide fluorescent probe and its preparation method and application
A hydrogen peroxide and fluorescent probe technology, applied in the field of chemical analysis and detection, can solve the problems of slow response, small Stokes shift value, complex synthesis, etc., and achieve fast response speed, large Stokes shift value, highly responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Compound A (0.195g, 1mmol) and 4-bromomethylphenylboronic acid pinacol ester (0.296g, 1.0mmol) were dissolved in 50mL of acetonitrile, potassium carbonate (0.690g, 5.0mmol) was added, and the reaction was carried out under reflux at 85°C for 8h . After the reaction, the solvent was distilled off under reduced pressure, then dissolved in dichloromethane and methanol, and purified by column chromatography to obtain 0.357 g of near-red solid powder (87% yield). After the product is analyzed by proton nuclear magnetic resonance spectrum, its structural formula is as shown in formula (1):
[0043]
[0044]Wherein the structural formula of compound A is as shown in formula (2):
[0045]
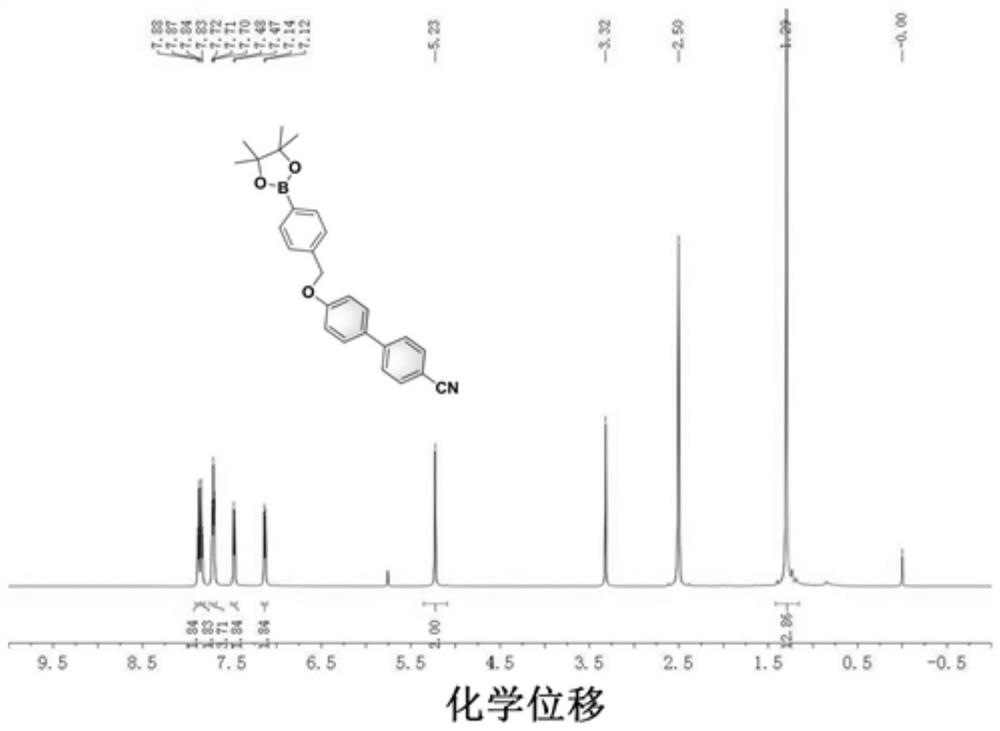
[0046] The H NMR spectrum is as figure 1 As shown, the description of the spectrum is as follows:
[0047] 1 H NMR (400MHz, DMSO): δ7.88–7.83(m,4H),7.72–7.70(m,4H),7.48–7.47(m,2H),7.14–7.12(m,2H),5.23(s, 2H), 1.29(s,12H).MS: m / z, theoretical value: [M+H] + 412.21; Calculated: 412...
Embodiment 2
[0049] Compound A (0.195g, 1mmol) and 4-bromomethylphenylboronic acid pinacol ester (0.296g, 1.0mmol) were dissolved in 50mL of acetonitrile, sodium carbonate (0.530g, 5.0mmol) was added, and the reaction was refluxed at 40°C for 8h . After the reaction, the solvent was distilled off under reduced pressure, then dissolved with dichloromethane and methanol, and purified by column chromatography to obtain 0.243 g of near-red solid powder (yield 85.9%).
Embodiment 3
[0051] Compound A (0.195g, 1mmol) and 4-bromomethylphenylboronic acid pinacol ester (0.296g, 1.0mmol) were dissolved in 50mL of acetonitrile, potassium carbonate (0.690g, 5.0mmol) was added, and the reaction was refluxed at 85°C for 12h . After the reaction, the solvent was distilled off under reduced pressure, then dissolved in dichloromethane and methanol, and purified by column chromatography to obtain 0.318 g of near-red solid powder (yield: 77%).
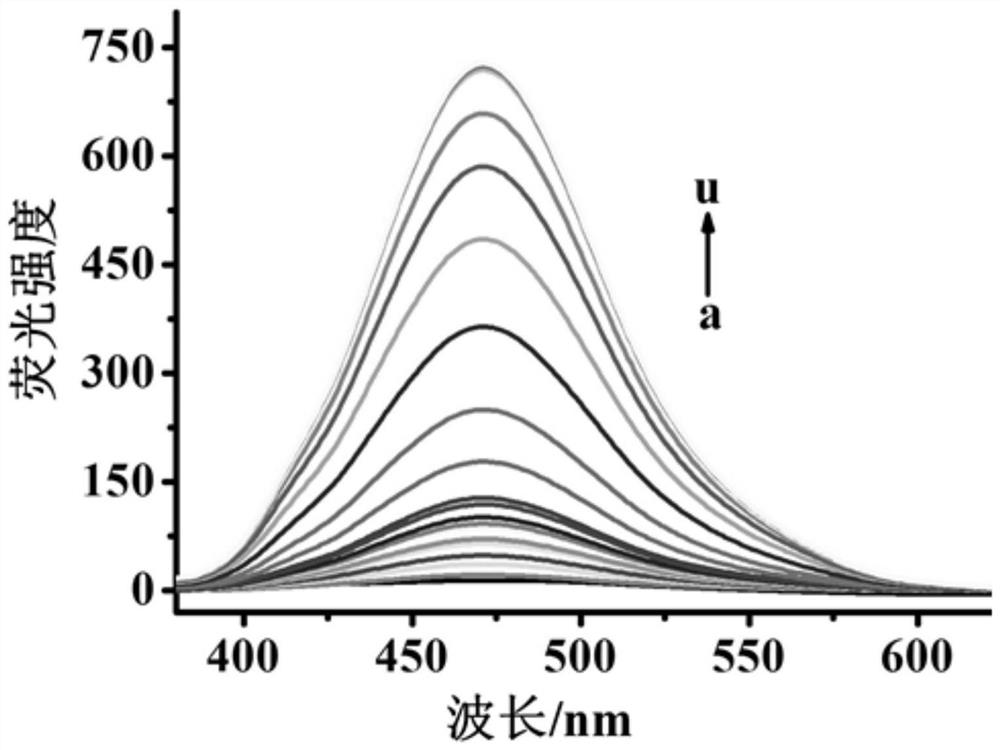
[0052] Fluorescent detection of hydrogen peroxide with fluorescent probes for hydrogen peroxide:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com