Gastric cancer pharmaceutical composition and application thereof
A composition and drug technology, applied in the field of immunology, can solve the problem of patients' response rate reduction to previous therapy, and achieve significant cytotoxicity and effective tumor control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Preparation of anti-PSCA CAR-T cells
[0077] (1) Construction of CAR molecular lentiviral vector
[0078] Gene synthesis of a CAR molecule composed of GM-CSF signal peptide, anti-PSCA single chain variable fragment (single chain variable fragment, scFv), hinge region, CD28, CD3ζ and DAP10, the nucleic acid sequence is shown in SEQ ID NO: 8-13, and A recognition site for the restriction endonuclease Pme1 is added to the C-terminus of the CAR molecule, a recognition site for the restriction endonuclease Spe1 is added to the N-terminus, and the synthetic gene sequence is temporarily connected to the PUC57 vector;
[0079] The linearized CAR molecule and the Pwpxld-eGFP sequence were respectively obtained by double enzyme digestion with Pme1 and Spe1, and the above fragments were recovered after agarose gel electrophoresis;
[0080] Using TAKARA's solution 1, connect the linearized CAR molecule with Pwpxld-eGFP to obtain a circular plasmid;
[0081] Transform the...
Embodiment 2
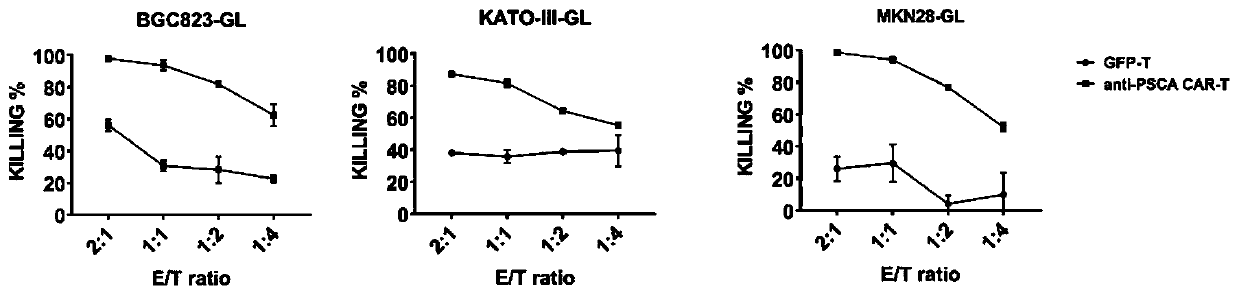
[0096] Example 2 In vitro detection of the killing ability of anti-PSCA CAR-T cells on gastric tumor cells
[0097] (1) Anti-PSCA CAR-T cells and GFP-T cells prepared in Example 1 were co-incubated with target cells BGC-823-GL, KATO-Ⅲ-GL and MKN28-GL at different ratios for 18-24 hours , using a luciferase substrate to detect the ratio of living cells, the effect-to-target ratio of CAR-T cells and tumor cells is 2:1, 1:1, 1:2 or 1:4, and the amount of target cells is 1×10 4 per well (96-well plate), the incubation volume is 200 μL;
[0098] (2) After the co-incubation is completed, take 100 μL of the incubation supernatant and temporarily store it at -20°C to detect the secretion of cytokines;
[0099] (3) After diluting 100× fluorescein sodium salt to 2× with PBS, add 100 μL per well to the remaining cell solution in step (2), mix well while avoiding the generation of air bubbles, and incubate at 37°C for 5-10 minutes. The RLU (relative light unit) was detected by a fluores...
Embodiment 3
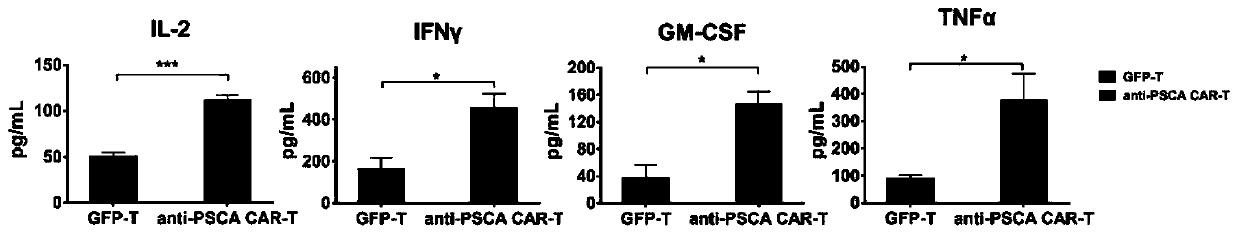
[0103] Example 3 In vitro detection of cytokine secretion ability of anti-PSCA CAR-T and GFP-T
[0104] (1) Take out the culture supernatant temporarily stored at -20°C in step (2) of Example 2, and dilute the corresponding ratio after thawing;
[0105] (2) The contents of IL-2, IFN-γ, GM-CSF and TNF-α in the supernatant were detected by cytokine detection kit (ebioscience).
[0106] The result is as figure 2 As shown, anti-PSCA CAR-T has significantly improved secretion capacity of IL-2, IFN-γ, GM-CSF and TNF-α compared with GFP-T.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com