Method for electrochemically synthesizing sequence-controllable coordination metal polymer
A technique of coordinating metals and synthesizing sequences, applied to electrodes, electrolytic processes, electrolytic components, etc., can solve the problems of long time consumption, low efficiency, and prone to side reactions, etc., and achieve the effect of short synthesis cycle and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
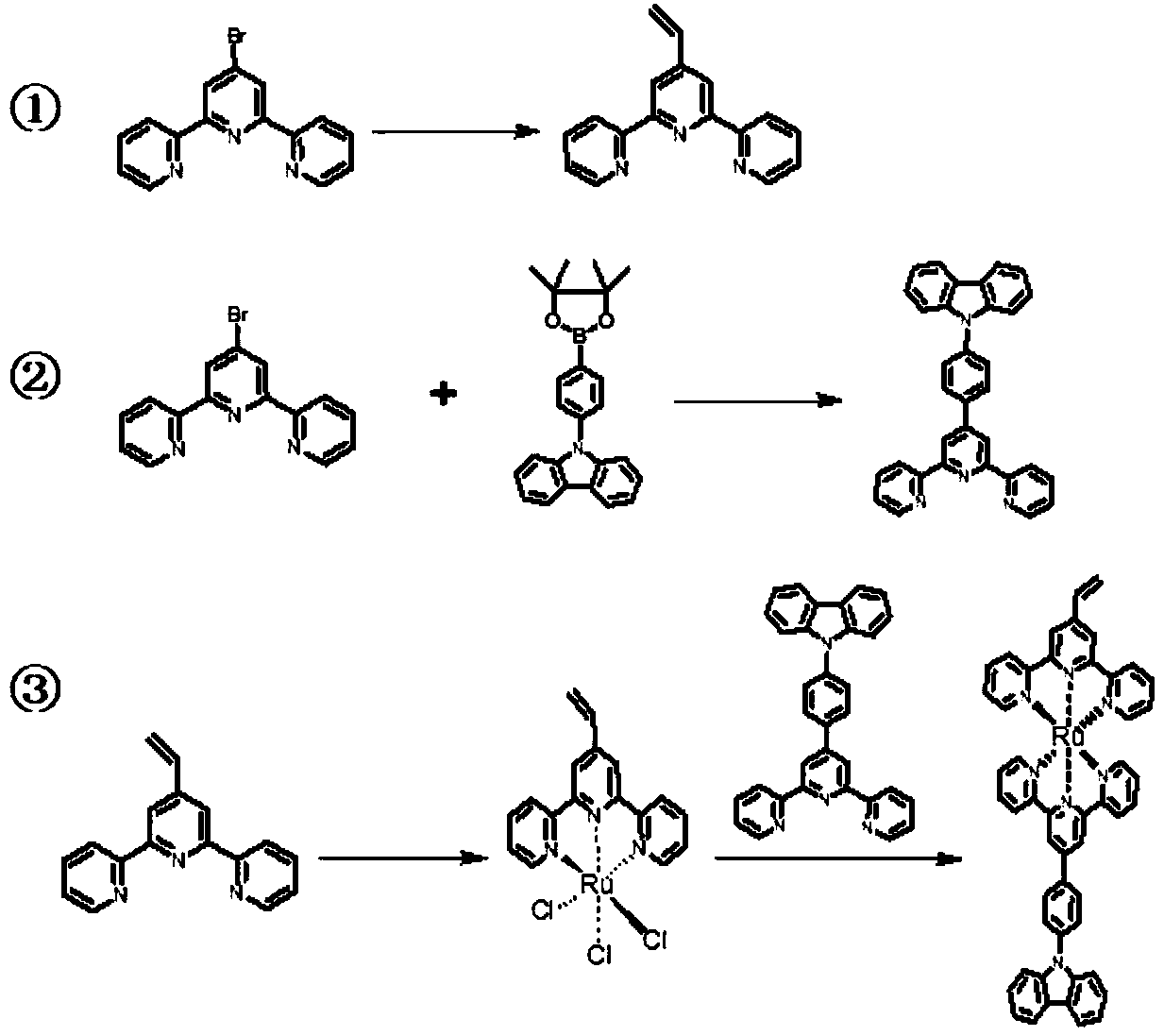
[0026] Example 1: Synthesis of monomers with electroactive units
[0027] Just take the synthesis of the following structural monomer as an example, the active core unit of electrochemical oxidation is carbazole, the active core unit of electrochemical reduction is vinyl substituted by benzene ring, and the central element unit of complex metal core is ruthenium.
[0028] Basic synthetic route:
[0029]
[0030] 1. Synthesis of 4'-vinyl-[2,2'; 6',2"] terpyridine
[0031] 200mg (0.64mM) 4'-bromo-2,2':6',2"-terpyridine, 104mg (0.76mM) potassium vinyl trifluoroborate, 3mg (0.0128mM) palladium acetate, 10mg (0.04mM) three Phenylphosphine and 652mg (2mM) cesium carbonate were reacted in 10mL of tetrahydrofuran / water solvent with a volume ratio of 24 / 1 at 85°C for 48 hours. After the reaction was cooled, it was quenched with water, extracted with dichloromethane, dried over anhydrous magnesium sulfate, and Petroleum ether, dichloromethane, and ammonia water were used as eluents...
Embodiment 2
[0036] Example 2: Synthesis of Self-Assembled Molecules
[0037] Just take the synthesis of self-assembled molecules with the following structure as an example, the end group of the oxidation reaction is carbazole, the end group reacting with the working electrode is a phosphate group, and the central element unit of the complex is ruthenium.
[0038] Basic synthetic route:
[0039]
[0040] 1. Synthesis of [2,2'; 6',2"-terpyridine']-4-diethyl phosphite
[0041] 312mg (1mM) 4'-bromo-2,2':6',2"-terpyridine, 414.3mg (3mM) diethyl phosphite, 57.8mg (0.05mM) tetrakistriphenylphosphine palladium, 0.3mL ( 2mM) triethylamine was reacted in 30mL toluene solvent at 95°C for 12 hours. After the reaction was cooled, add water to quench the reaction, extract with dichloromethane, dry over anhydrous magnesium sulfate, use dichloromethane and methanol as eluent, and perform column chromatography Purification afforded a white solid (86% yield). 1 H NMR (400MHz, CDCl 3 ,25℃): δ(ppm)8.8...
Embodiment 3
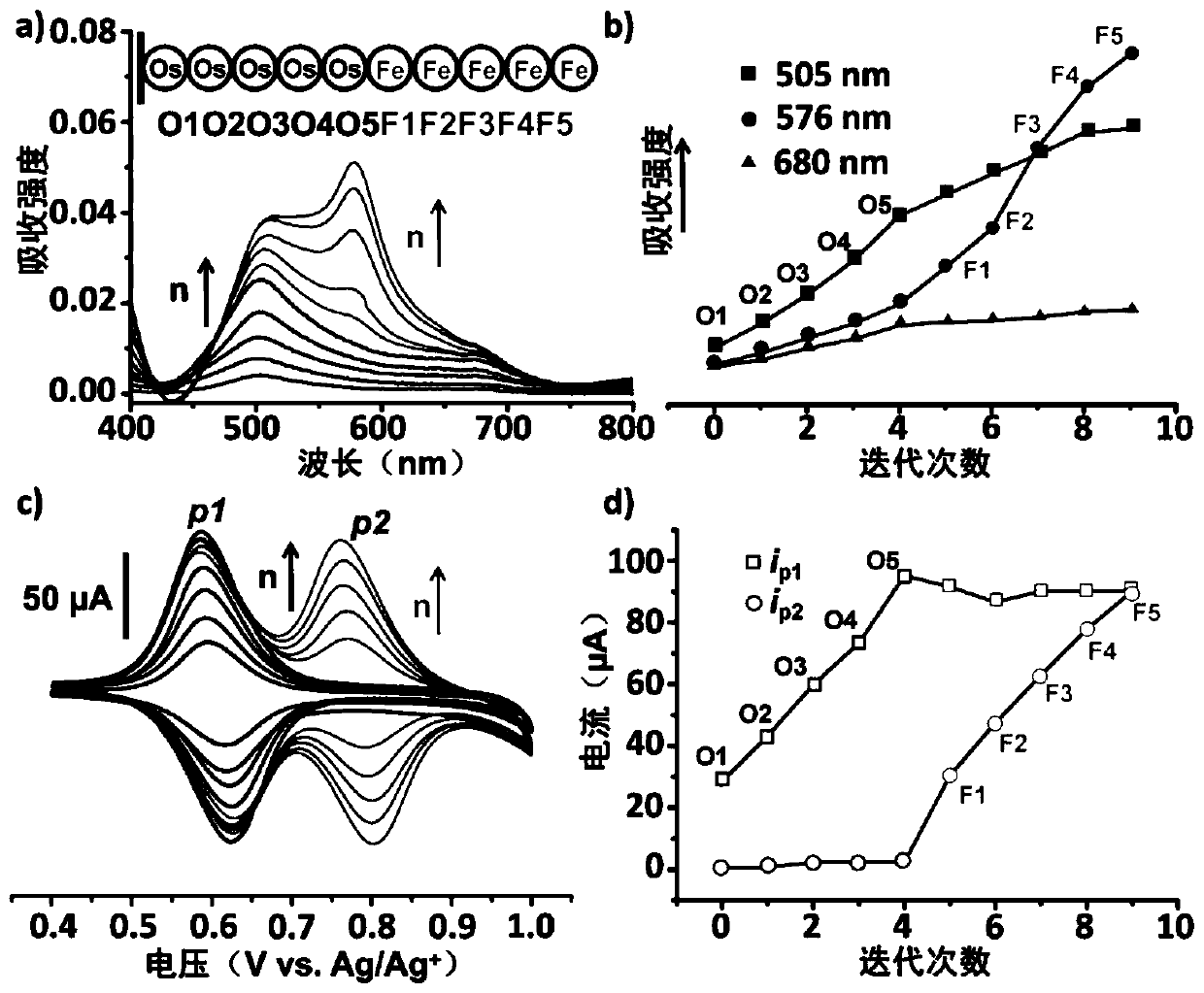
[0044] Example 3: Electrochemical synthesis of sequence-controllable coordination metal polymers
[0045] 1. Electrolyte preparation
[0046] The synthesized complex monomer was dissolved in the electrolyte solution, and the concentration of the compound was 0.5 mM. The supporting electrolyte is tetrabutylammonium hexafluorophosphate with a concentration of 0.1mol / L. The solvent is acetonitrile.
[0047] 2. Use of electrodes
[0048] (1) Working electrode:
[0049] Just take the self-assembled monolayer prepared by using indium tin oxide (ITO) electrodes on the assembly substrate as an example:
[0050] The ITO glass was soaked in 0.1mM self-assembled molecules / methanol solution for 12 hours in the dark, ultrasonically cleaned in an ethanol bath, the surface was rinsed with dichloromethane, and dried with nitrogen gas for later use.
[0051] (2) Counter electrode: platinum wire electrode
[0052] (3) Reference electrode: silver-silver chloride electrode
[0053] 3. Poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com